Clinical Evaluation Report Template Mdr
Here are some of the images for Clinical Evaluation Report Template Mdr that we found in our website database.

Clinical Evaluation Report Template Google Docs Word Template net

Clinical Evaluation Report (CER) Template Clinical Study Templates

Clinical Evaluation Report Template Mdr

Clinical Evaluation Report Template Mdr

Clinical Evaluation Report Template Mdr

Clinical Evaluation Plan Template MDR Best CER Plan

MDR Clinical Evaluation Report merging MEDDEV and MDCGs

MDR Clinical Evaluation Report merging MEDDEV and MDCGs

Clinical Evaluation Report and Template with Example
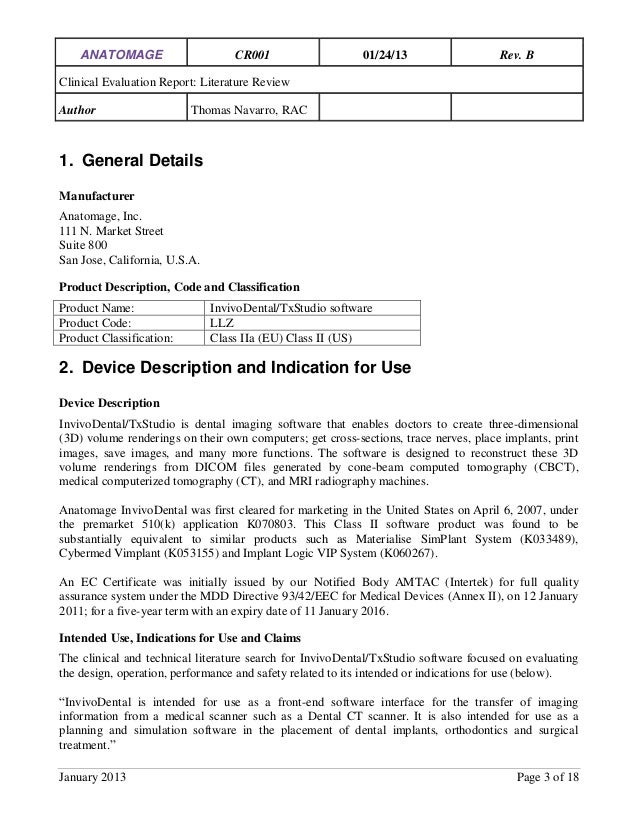
Clinical Evaluation Report Template Mdr

Clinical Evaluation Report Template Mdr

MDR Compliant Clinical Evaluation Report Template

MDR Compliant Clinical Evaluation Report Template

MDR Clinical Evaluation AKRA TEAM

Ivdr Performance Evaluation Plan Template Fill Online Printable

Clinical Evaluation Report Template
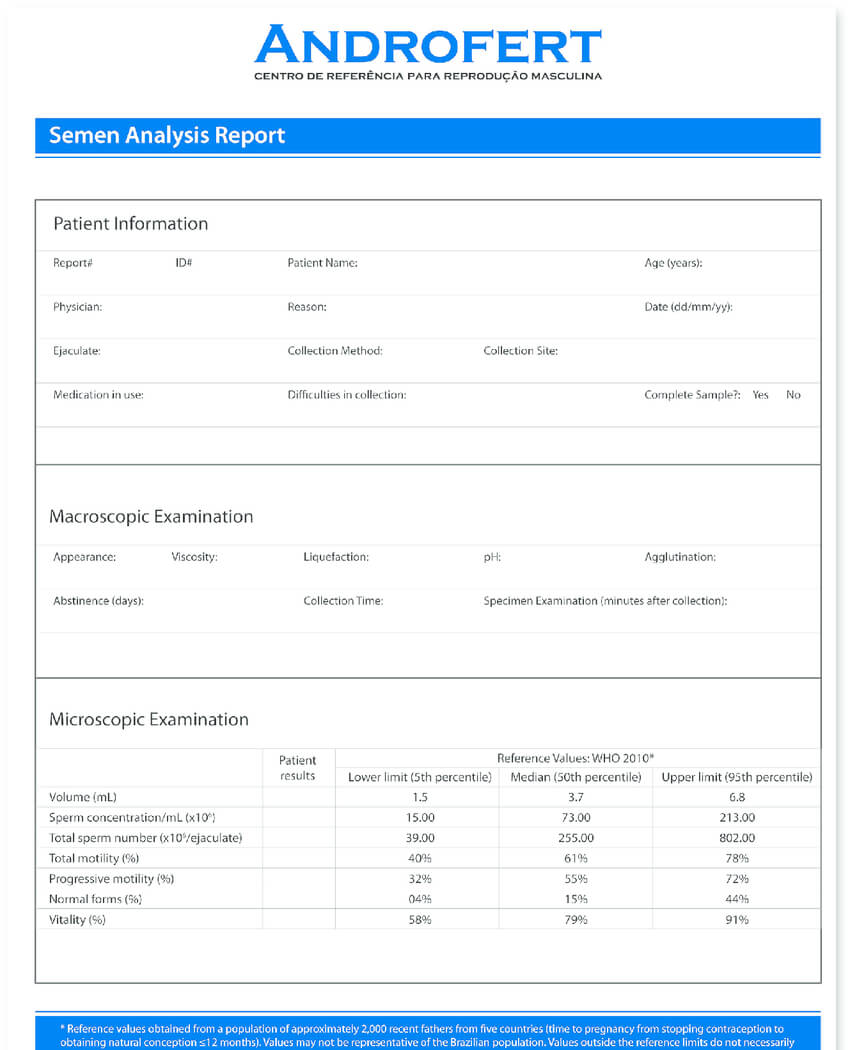
Clinical Evaluation Report Template
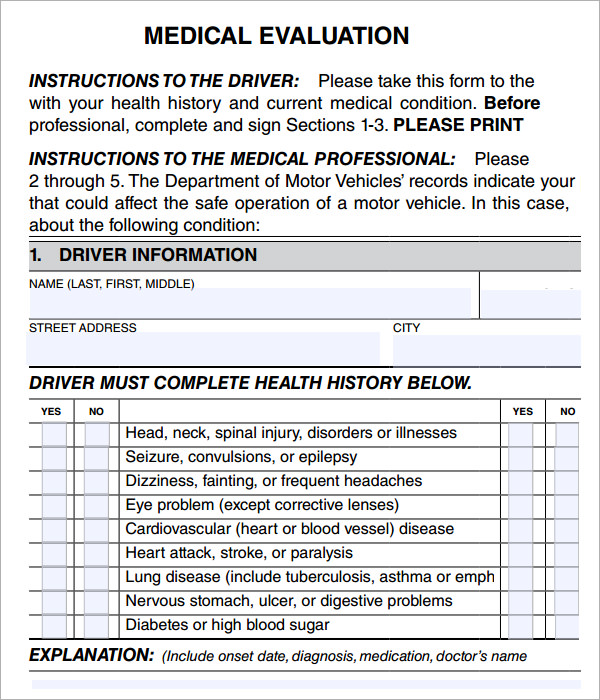
Clinical Evaluation Report Template

Clinical Evaluation Report Template

Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co
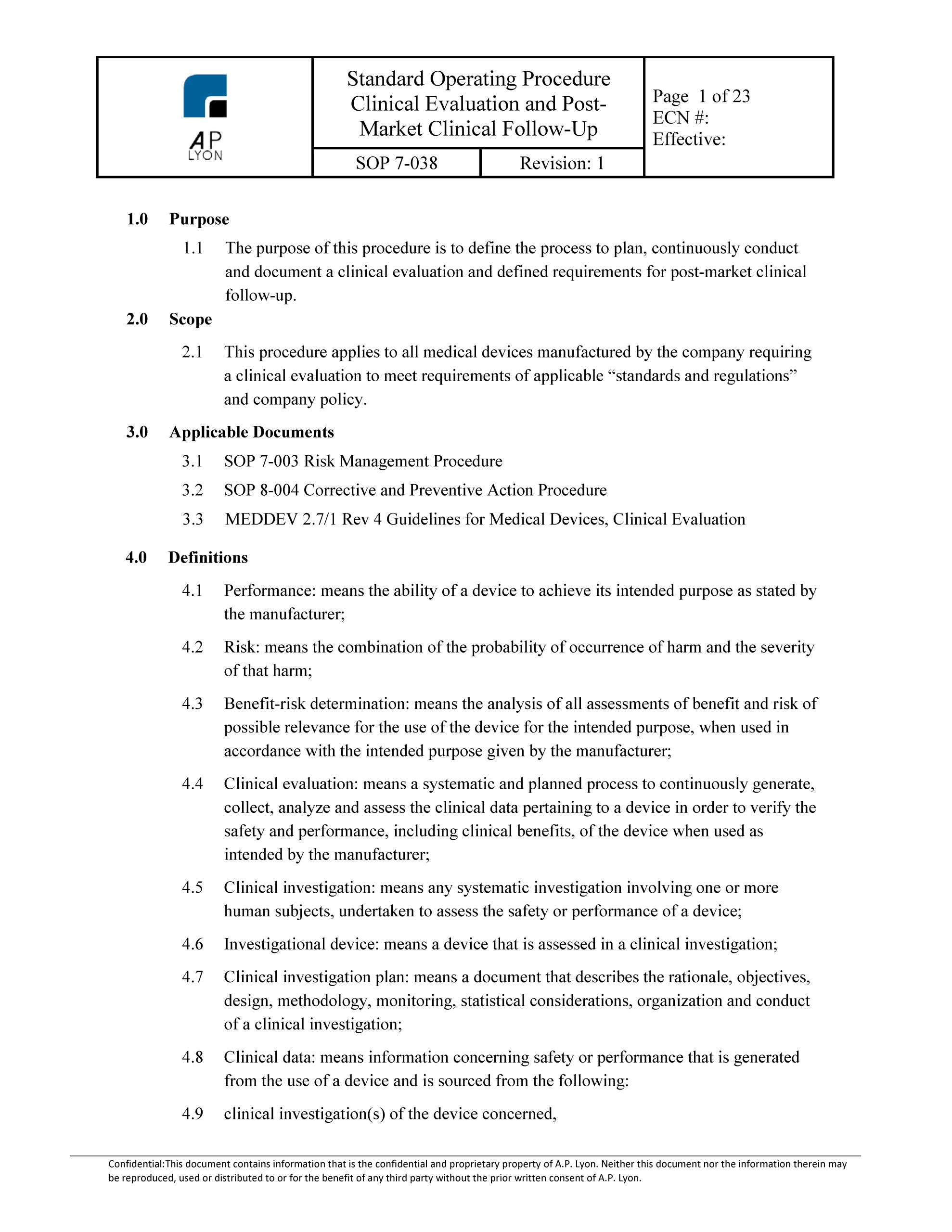
Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co
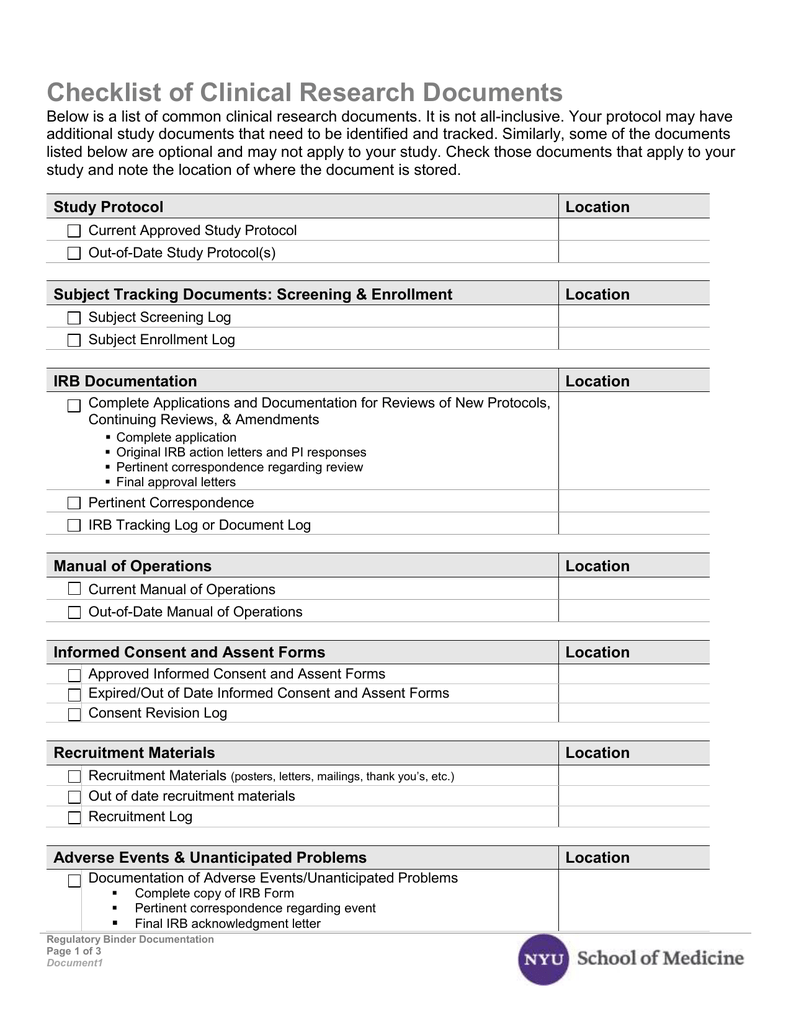
Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co

Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co

Create a Clinical Evaluation Report Under MEDDEV MDR

Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co
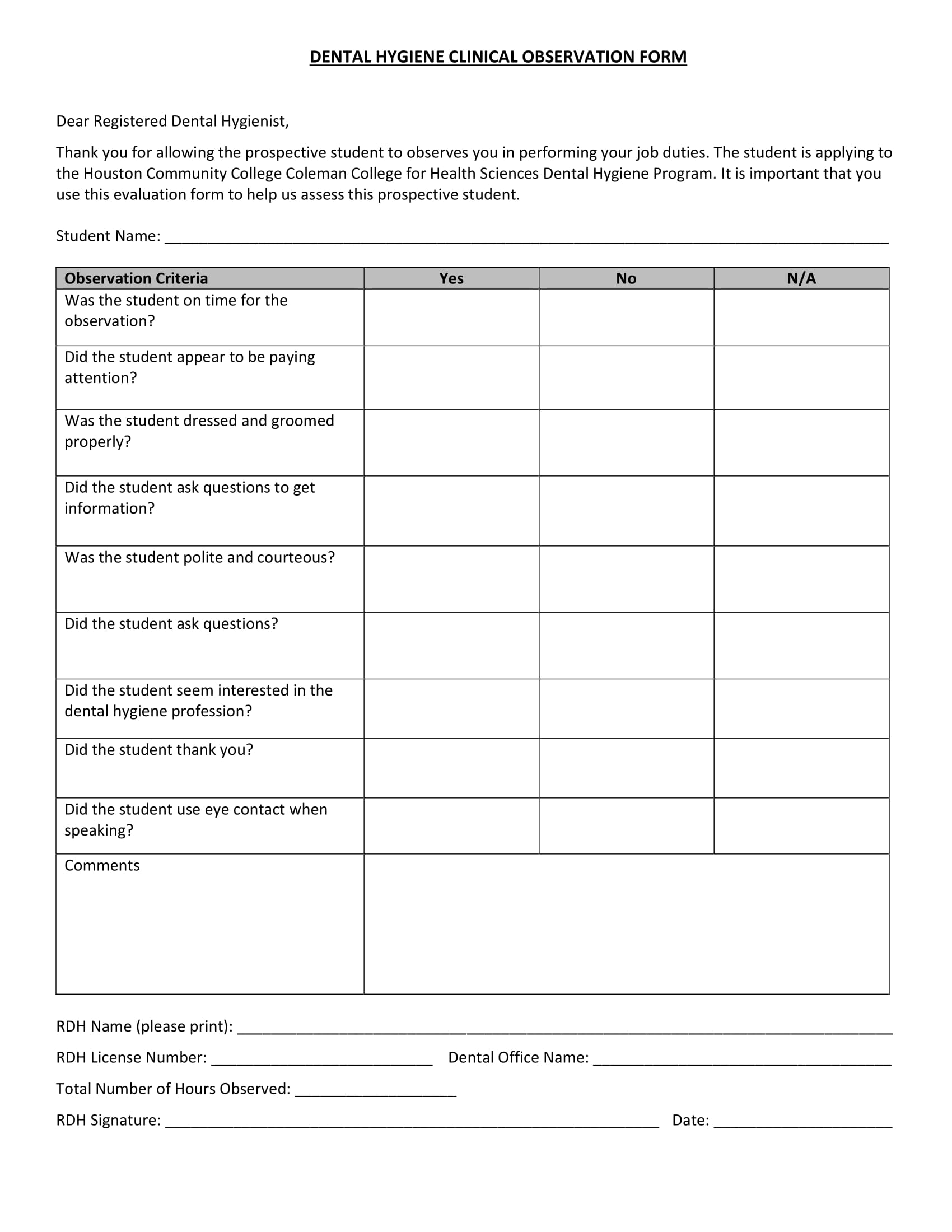
Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co

Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co

Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co

Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co

Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co

PPT Clinical Evaluation Report MDR Requirements PowerPoint

PPT Clinical Evaluation Report MDR Requirements PowerPoint

PPT Clinical Evaluation Report MDR Requirements PowerPoint

MDR Clinical Evaluation Plan · MDlaw Information platform on European

Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co

Clinical Evaluation Report Template prntbl concejomunicipaldechinu gov co

Clinical Evaluation Report (CER) Under MDR
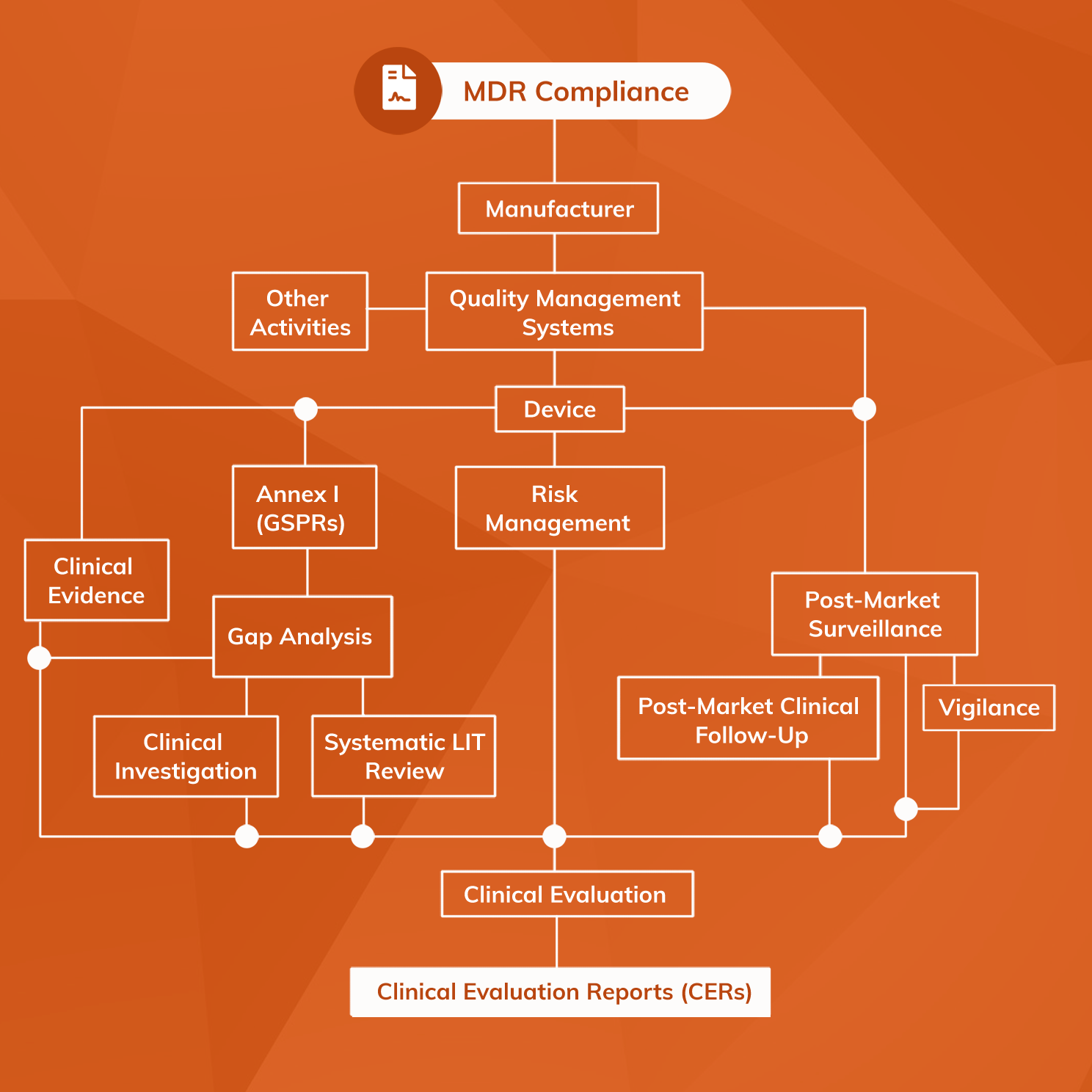
Clinical Evaluation Report writing for EU MDR
Clinical Evaluation Report for Medical Device Manufacturer
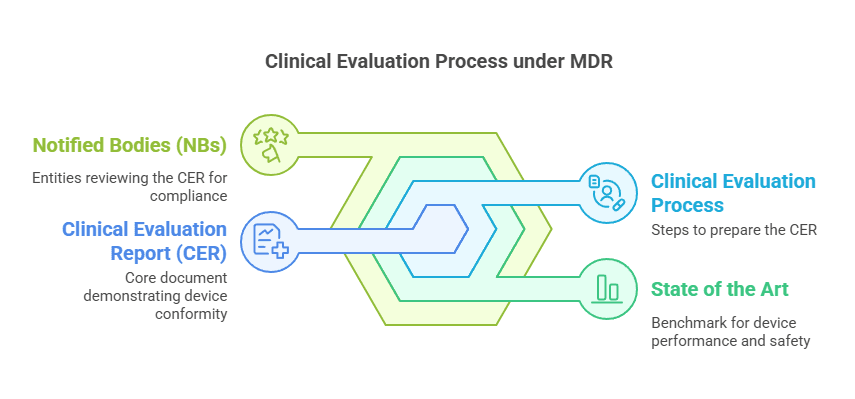
Clinical Evaluation under EU MDR 2017/745
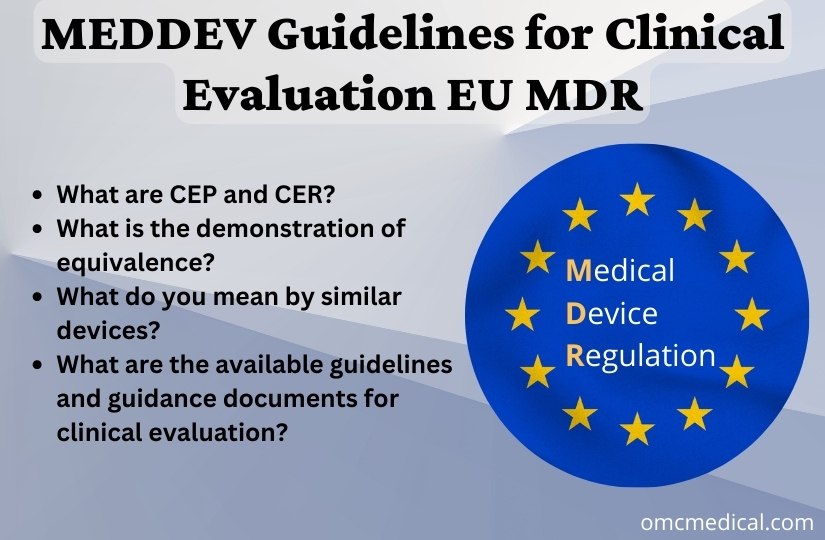
MEDDEV Guidelines for Clinical Evaluation EU MDR OMC Medical

Article 61 Clinical Evaluation in the EU MDR OMC Medical

Clinical Evaluation Plan Template

Medical Report Templates Documents Design Free Download Template net

Understanding key components of a Clinical Evaluation Report (CER
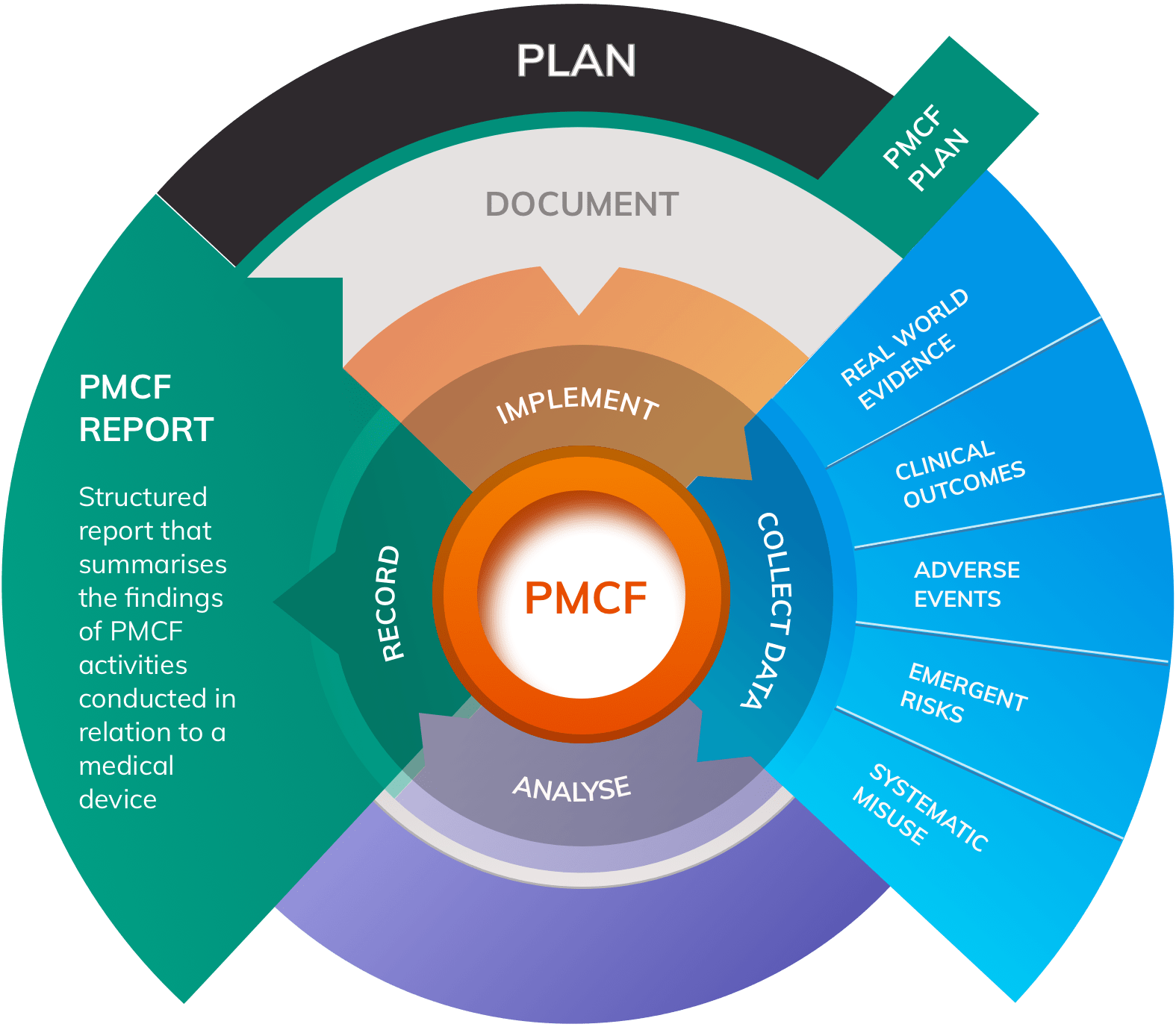
EU MDR Clinical Evaluation of medical devices

FREE Clinical Evaluation Report Template for MDR CER document writing

UDI Template (MDR)

Clinical evaluation report sample pdf: Fill out sign online DocHub

Clinical evaluation report sample pdf: Fill out sign online DocHub

Clinical evaluation report sample pdf: Fill out sign online DocHub

Clinical Evaluation Report Template: What to Include

Common Gaps in Clinical Evaluation Reports and Technical Documentation

Clinical Benefits Equivalence: EU MDR Clinical Evaluation Guide

Clinical Evaluation Report (CER): Everything You Need to Know

MDR clinical evaluation report: What is it and how to write it?
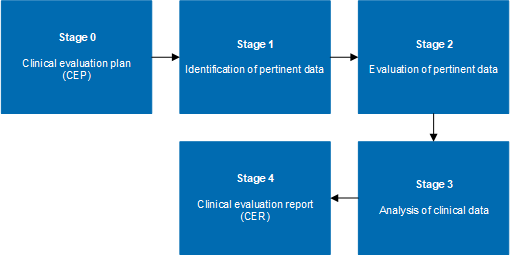
MDR clinical evaluation reports and plans for medical devices

EU MDR Templates CER PSUR PMCF Clinical Evaluation

Clinical Evaluation of Medical Devices tracekey solutions GmbH
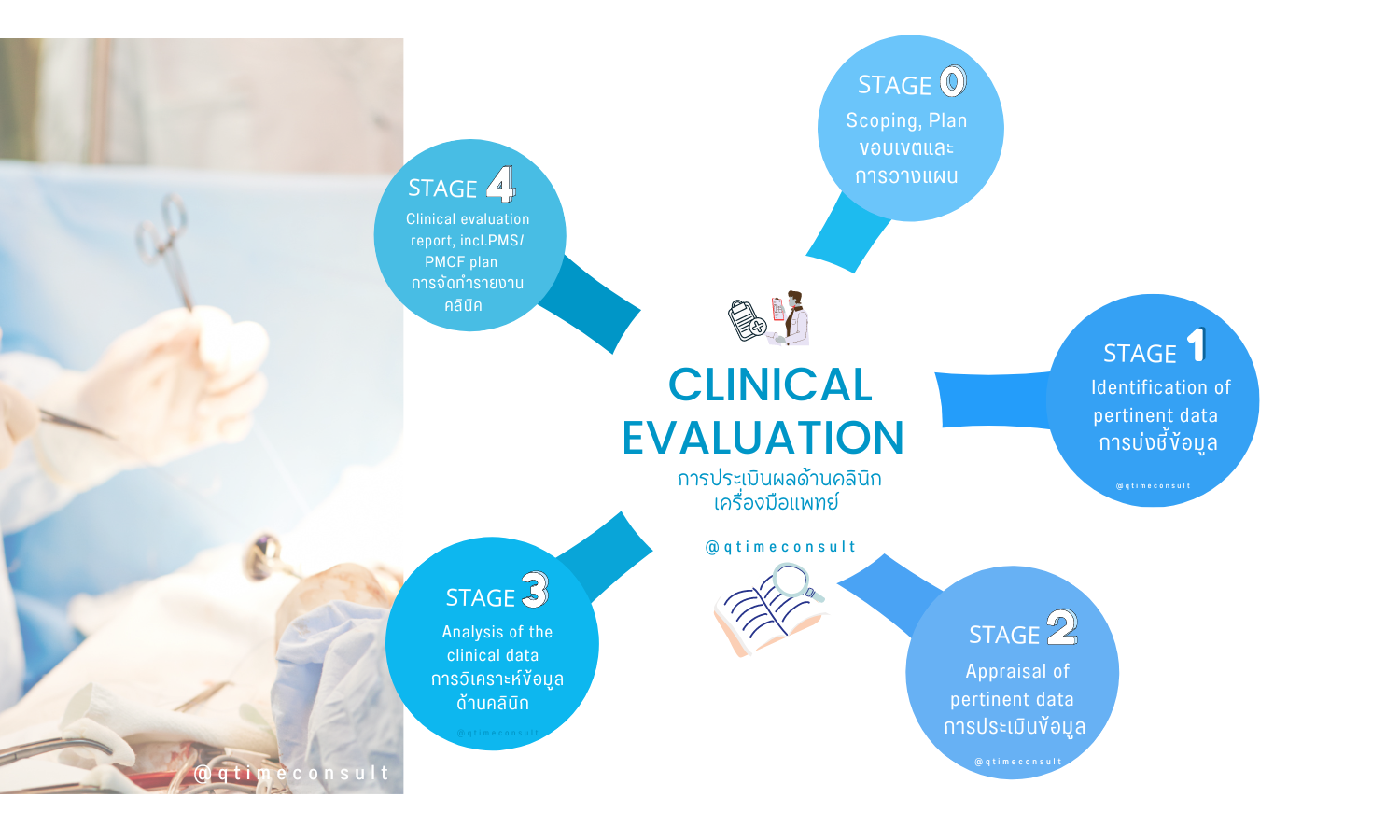
Clinical Evaluation for Medical Device