Device Master Record Template
Here are some of the images for Device Master Record Template that we found in our website database.

Device Master Record Index PDF Sterilization (Microbiology

Device Master Record: Overview of FDA Requiements

Device Master Record Template
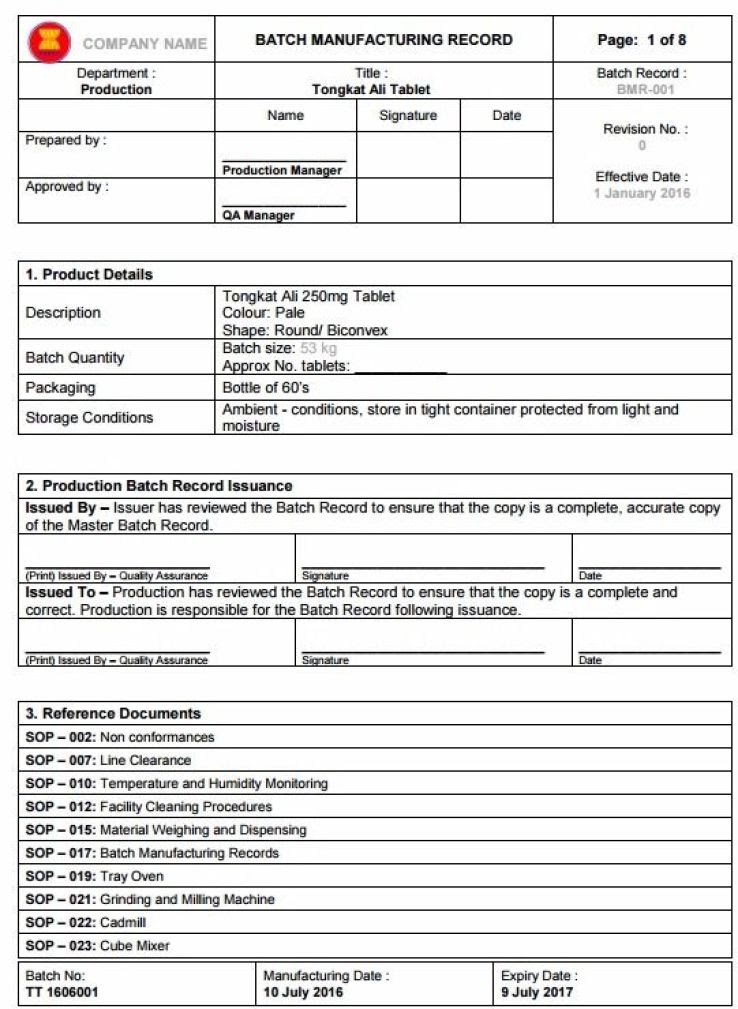
Device Master Record Template
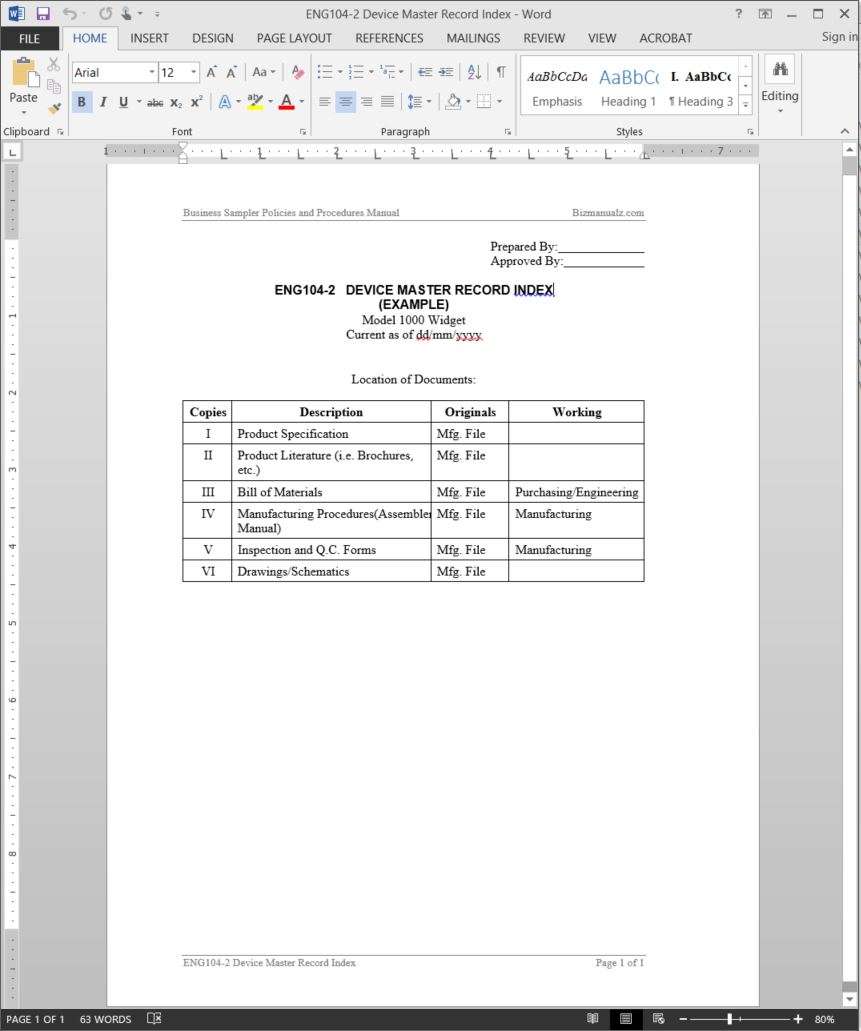
Device Master Record Template

Device Master Record Template

Device Master Record Template
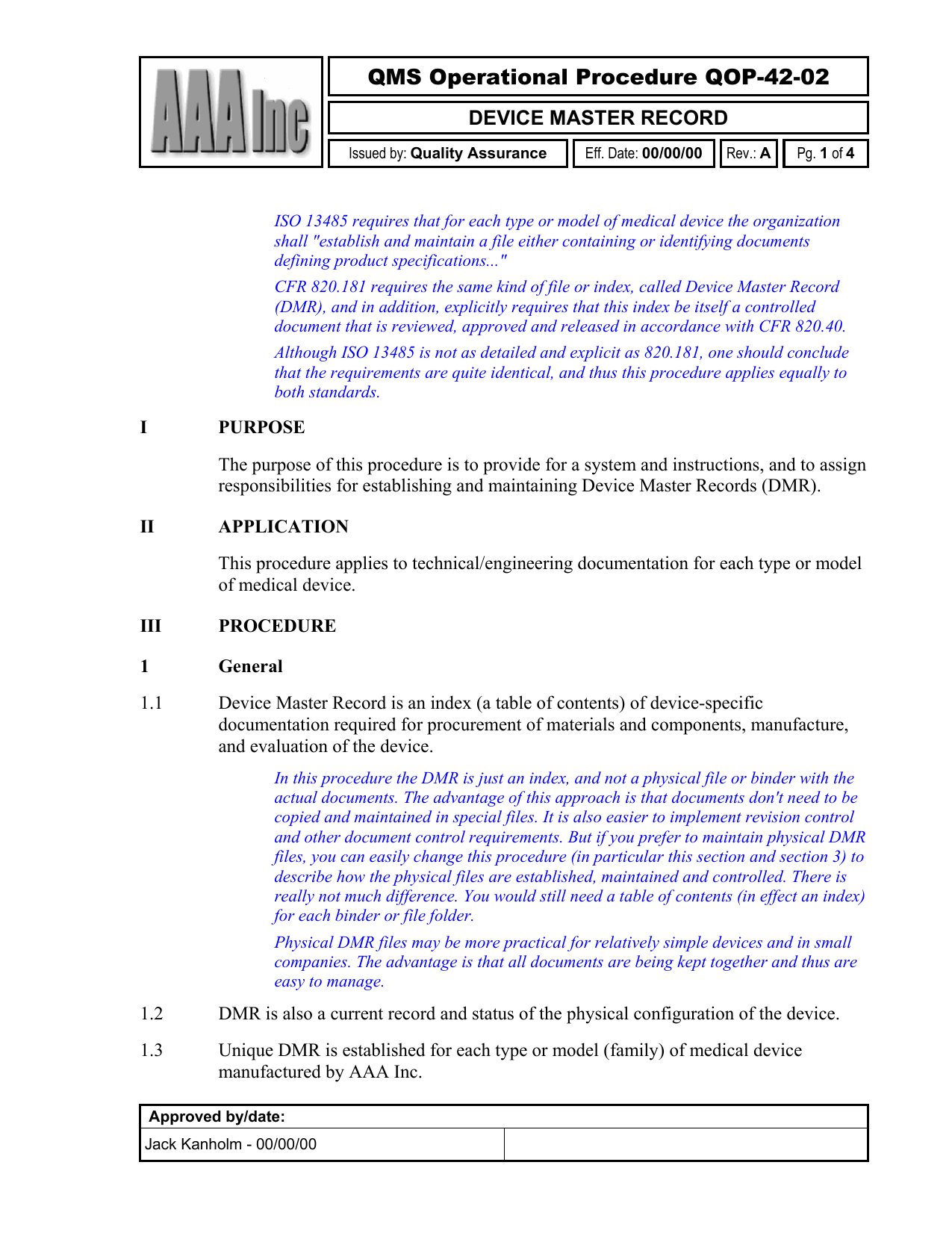
Device Master Record Template

Device Master Record Template

Device Master Record Template

Device Master Record
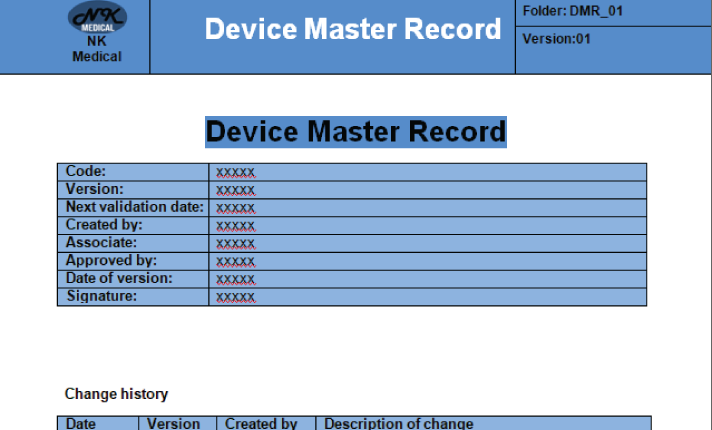
Device Master record meddevregulation com
%20Checklist.png?width=450&name=Device%20Master%20Record%20(DMR)%20Checklist.png)
DMR Checklist Free Download

Device Master Records (DMR) for Medical Devices (2023)

Device History Record Template
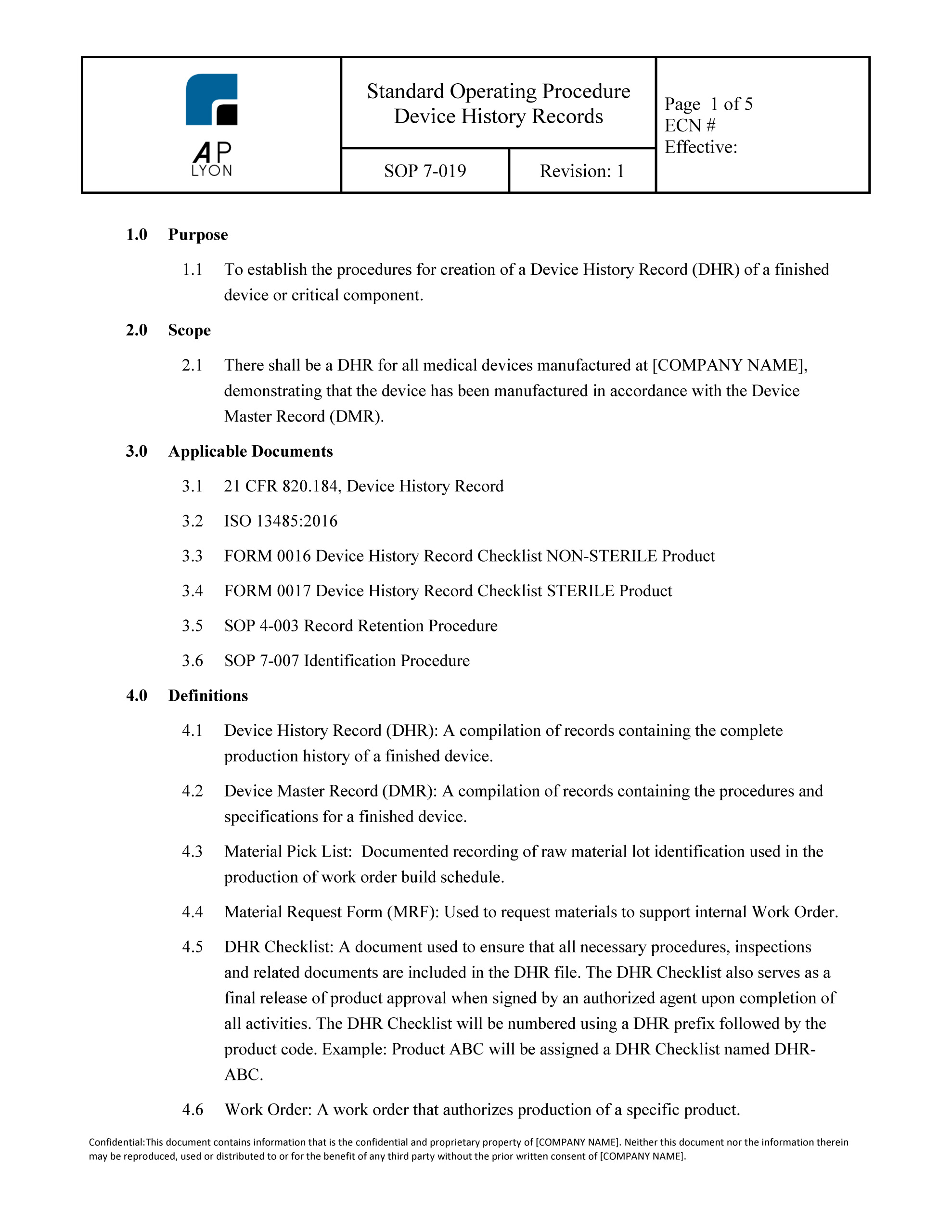
Device History Record Template

Device Master Record Index Template

What is Device Master Record (DMR)? Greenlight Guru

Device Master Record Index Template Word
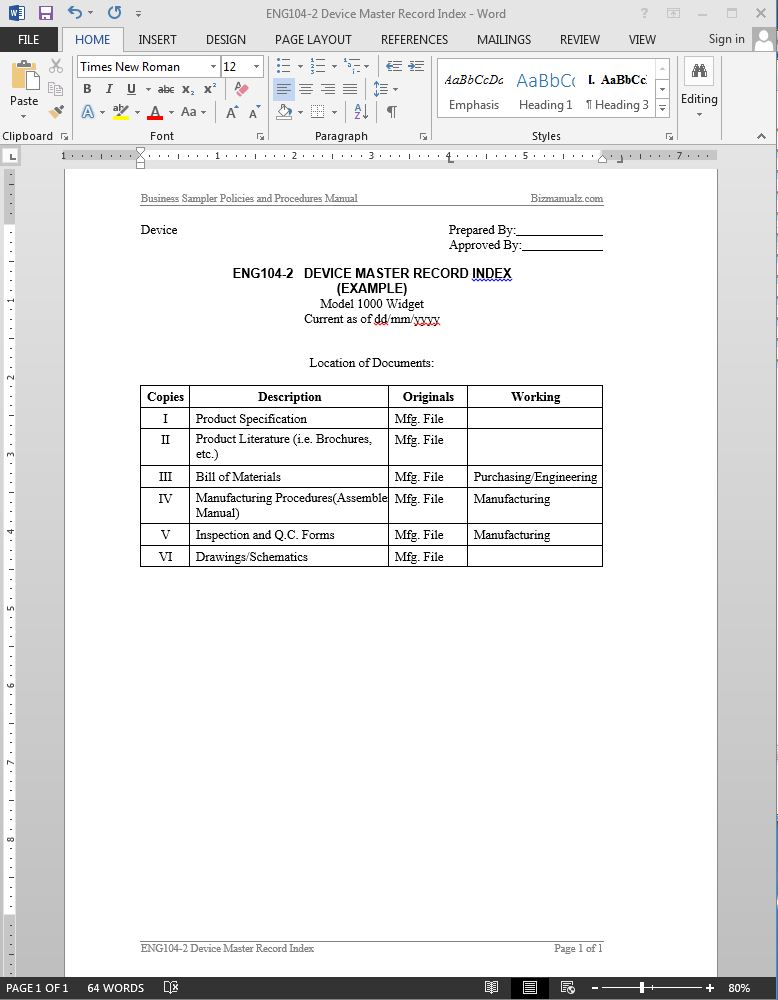
Device Master Record Index Template Word

Device Master Record Index Template Word

Device Master Record Index Template Word

Medical Device DTF DMR Remediation Services Celegence
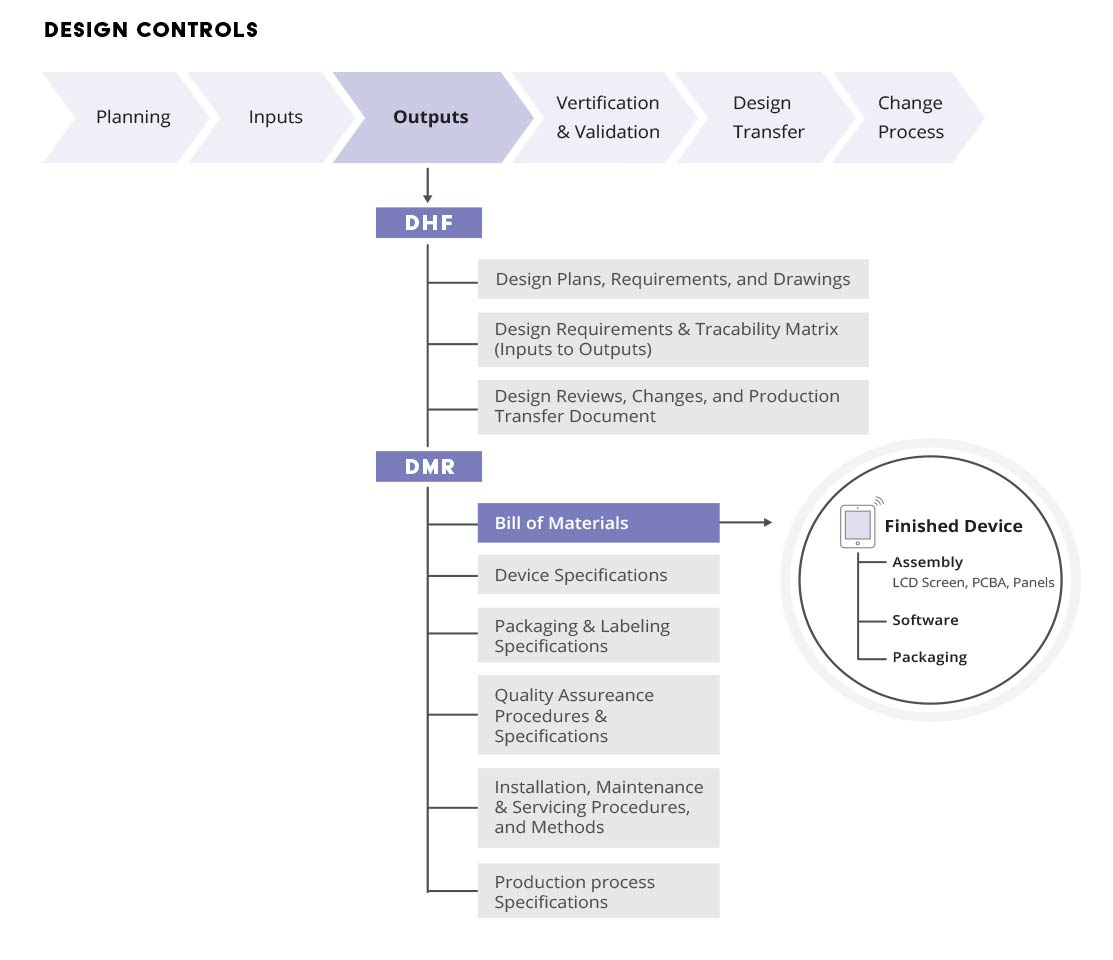
Managing The Device Master Record (DMR) Arena

Device Master Record (DMR): What It Is Why It Matters

Master Batch Record Template

Master Batch Record Template
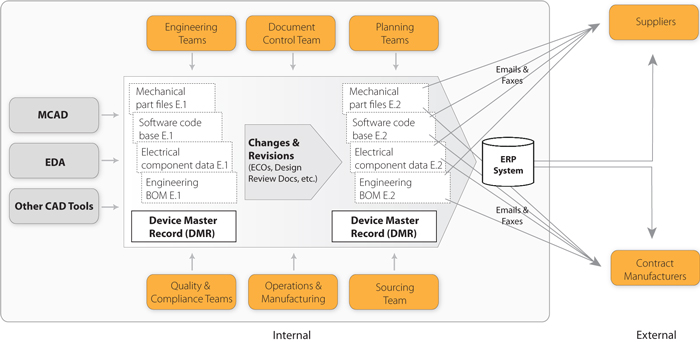
Maintaining the Device Master Record Arena Solutions

B Device Master Record Example 1 DEVICE MASTER RECORD EXAMPLE 1 No

Elements of Device Master Record (DMR) TS Quality Engineering

Index of /Cove Premium/DMR Device Master Record Procedure Example/

Index of /Cove Premium/DMR Device Master Record Procedure Example/

Device Master Record Procedure Template Word

Understanding the Importance of Device Master Record eLeaP Quality

Understanding the Importance of Device Master Record eLeaP Quality

Understanding the Importance of Device Master Record eLeaP Quality
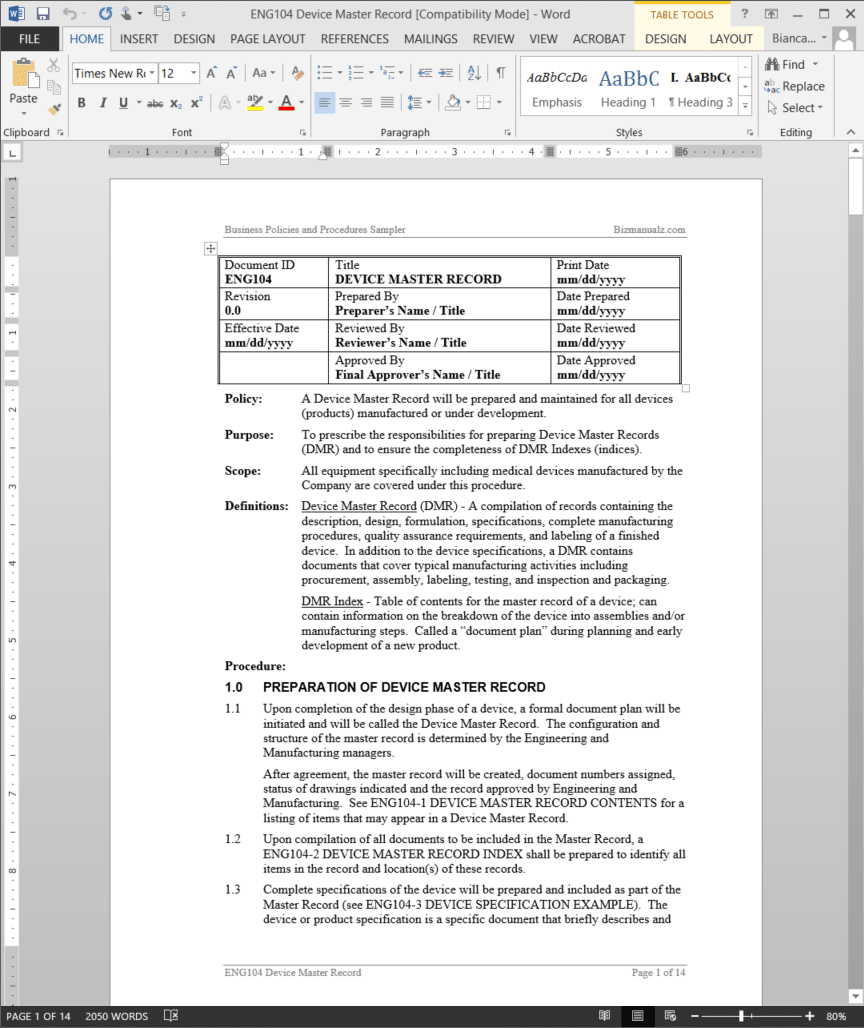
Device Master Record Procedure Template Word

Device History Record Template Fill Online Printable Fillable

Device History Record Procedure

Design master record design history file recipesplm

Medical Device Design History File Template prntbl

Device master record template pdf: Fill out sign online DocHub

Device master record template pdf: Fill out sign online DocHub

Device Master Record (DMR) Index Template 4EasyReg

Design History File (DHF) vs Device Master Record (DMR) vs Device
types of medical documents Okomeds

Device Master Record Index: Sl No Document No Device Specifications

Design History File Optimization Sequence Group
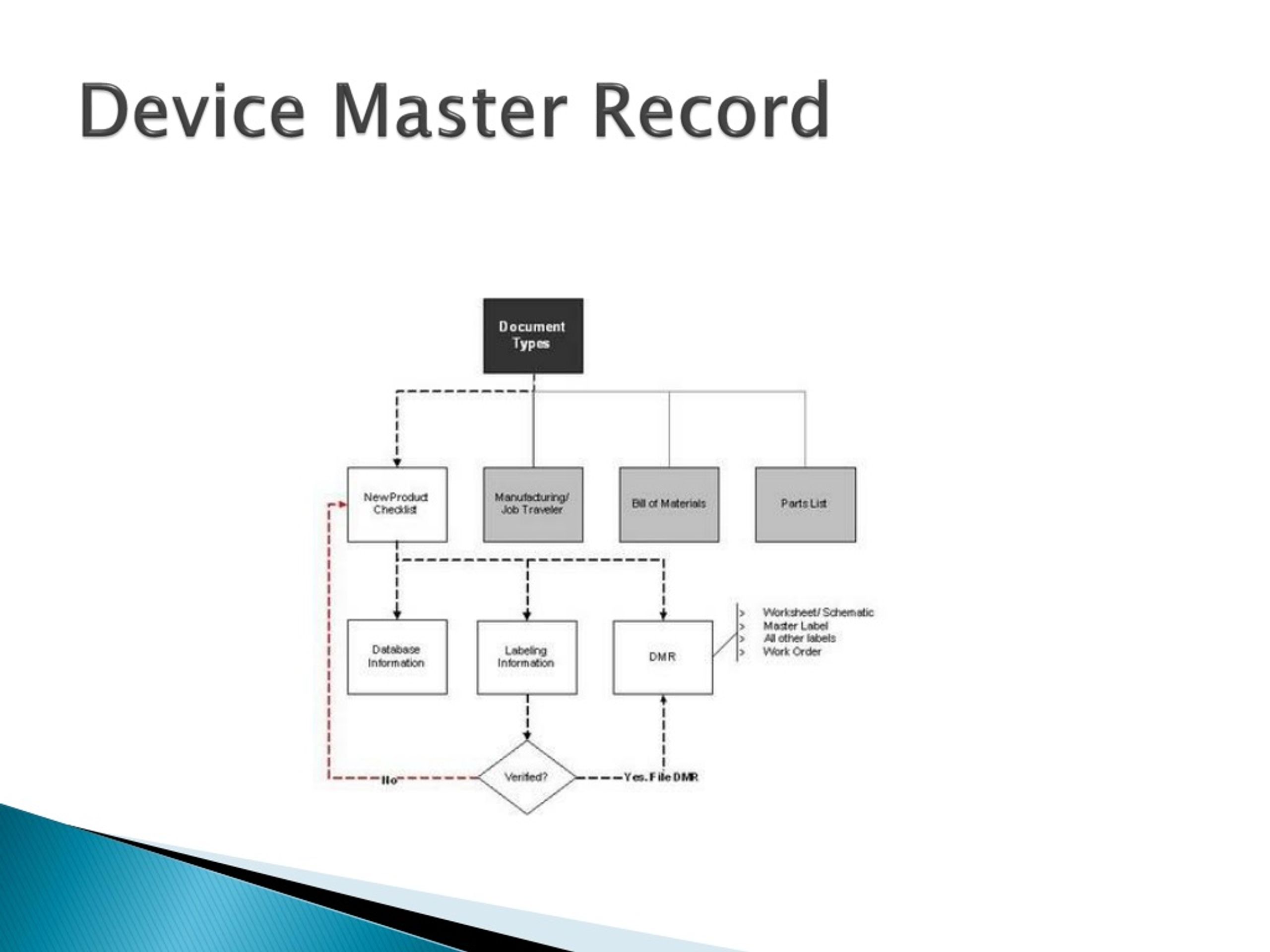
PPT Design Documentation PowerPoint Presentation free download ID

PPT Design Documentation PowerPoint Presentation free download ID
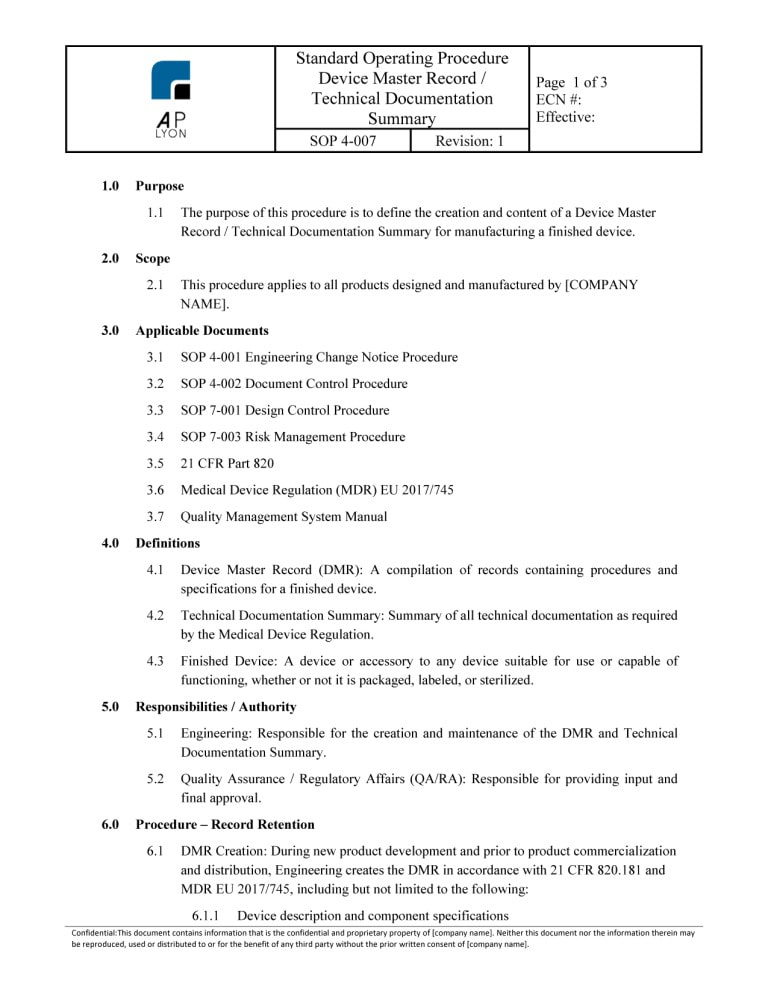
ISO 13485 Document Control Procedure Bundle

PPT Design Documentation PowerPoint Presentation free download ID

Design History Files: Everything You Should Know

Images of デバイスファイル JapaneseClass jp