Eu Mdr Certification Course
Here are some of the images for Eu Mdr Certification Course that we found in our website database.

European Union (EU) Definition Flag Purpose History Members

European Commission

Flags Of European Union

Map of the European Union Mappr
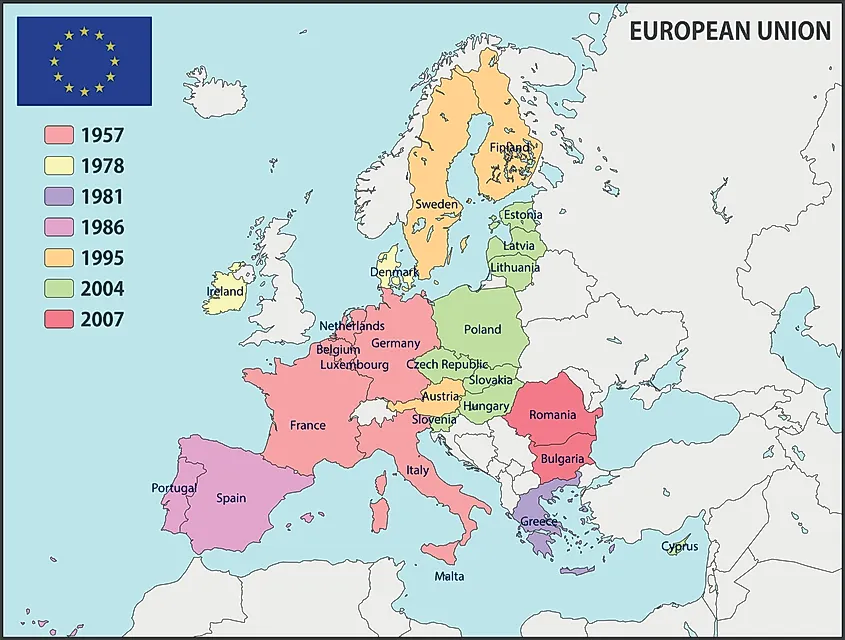
European Union WorldAtlas

European Union (EU) Definition Forexpedia™ by Babypips com
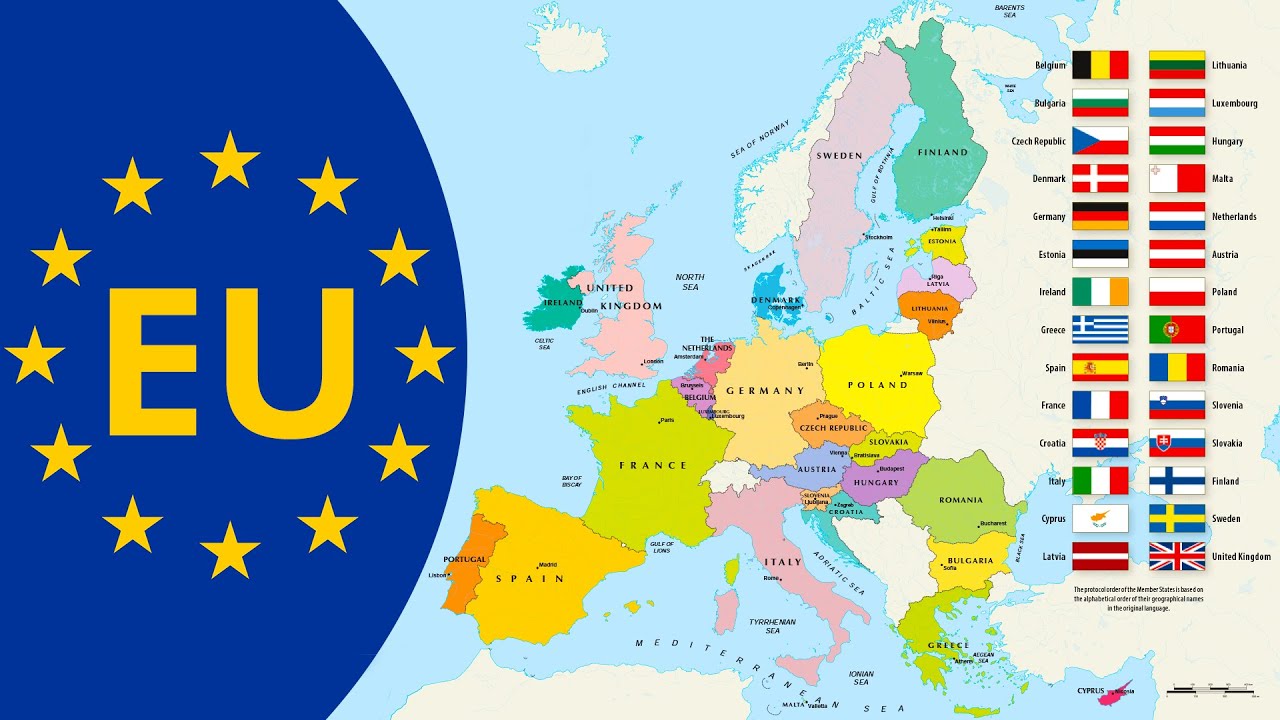
Countries of the European Union 2019 EU Member States with Flags

European Union Flag Wallpapers Top Free European Union Flag

European Union Wallpapers Wallpaper Cave

European Union

Vlajka Evropské unie Consilium

European Union Flag PNGs for Free Download