Eu Mdr Technical Documentation Template
Here are some of the images for Eu Mdr Technical Documentation Template that we found in our website database.

EU MDR Readiness Checklist PDF Verification And Validation

Technical Documentation Checklist For Medical Devices According To MDR

EU MDR Kit 4EasyReg

EU MDR Technical Documentation Kit 4EasyReg

MDR Technical Documentation Thema Med
EU MDR Technical Documentation Checklist (Annex II III) Blog
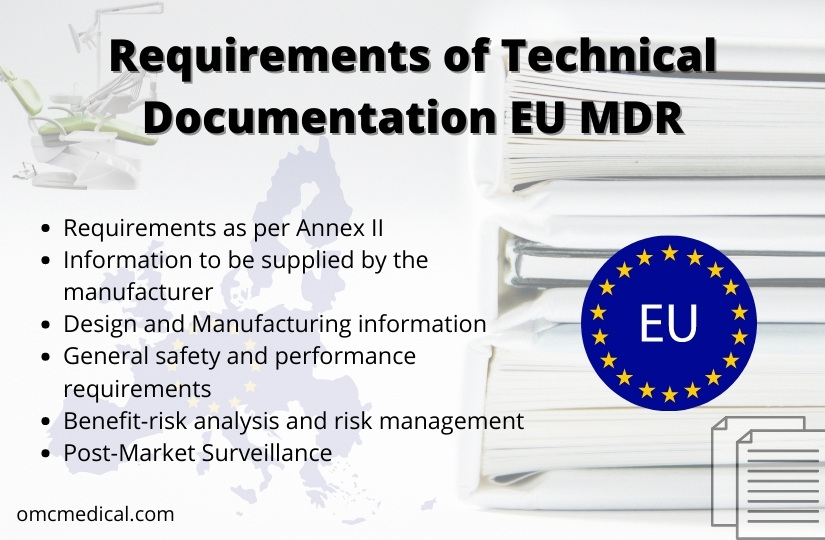
Requirements of Technical Documentation EU MDR OMC Medical

Technical Documentation under the EU MDR 2017/745

Understanding Notified Body Criteria for EU MDR Technical Documentation

Requirements Checklist for Technical Documentation According to EU MDR

Requirements Checklist for Technical Documentation According to EU MDR

Build eu mdr compliant technical documentation by Kenshawspecculo Fiverr

EU MDR Technical Documentation Checklist Celegence PDF Verification

FDA Vs EU MDR Technical Documentation Matrix EN PDF Federal Food

We help you under EU Technical Documentation

We help you under EU Technical Documentation

Technical File Template MDR (EU MDR 2017/745) QMSRegs com

Provide eu mdr technical documentation of medical devices by
Provide eu mdr technical documentation of medical devices by

BUNDLE Premium Pack EU MDR Technical File Easy Medical Device School

BUNDLE Premium Pack EU MDR Technical File Easy Medical Device School

BUNDLE Premium Pack EU MDR Technical File Easy Medical Device School

BUNDLE Premium Pack EU MDR Technical File Easy Medical Device School

BUNDLE Premium Pack EU MDR Technical File Easy Medical Device School

#eumdr #process #technicaldocumentation #registration #medicaldevices #

Free EU MDR Checklists PDF SafetyCulture

Free EU MDR Checklists PDF SafetyCulture

How to compile your technical documentation with success for regulatory