Fda Adcomm Calendar
Here are some of the images for Fda Adcomm Calendar that we found in our website database.

FDA

Update on FDA Reporting for Low Value Products

DOCMILTON

46 FDA Here #39 s another headline from last week: quot The FDA an Flickr

04/01/2008 05/01/2008 Medical Quack

Postapproval studies of drugs initially approved by the FDA on the

The Fate of Millions of People with PTSD Is in the Hands of the FDA

#rebaudiósido A BioQuizblog

Ukrainian Law Blog: FDA Authority Over Cosmetics: How Cosmetics Are Not

FDA Patient Safety News (June 2002) : Food and Drug Administration

FDA unter neuer Leitung

Class Action Investigations

Employees are the FIRST Line of Food Defense : Food and Drug

07/01/2008 08/01/2008 Medical Quack

06/01/2011 07/01/2011 Medical Quack

Živeti večno PULSE Magazin

Brawl at CP club during Pre Diwali celebration 1 hurt Nagpur Today

09/01/2011 10/01/2011 Medical Quack

FDA Patient Safety News (June 2002) : Food and Drug Administration

FDA Patient Safety News (November 2004) : Food and Drug Administration

FDA aprobă primul anticorp monoclonal biosimilar pentru tratarea cancerului

FDA Patient Safety News (November 2004) : Food and Drug Administration

Predicting Regulatory Product Approvals Using a Proposed Quantitative

FDA Patient Safety News (November 2004) : Food and Drug Administration

SCIENCE AND MISSION AT RISK: FDA #39 S SELF ASSESSMENT : Committee on

FDA Patient Safety News (May 2003) : Food and Drug Administration

Fred and the Voice of Food Safety: How to Avoid Food Borne Illness

Employees are the FIRST Line of Food Defense : Food and Drug

Cost benefit analysis and the FDA: measuring the costs and benefits of
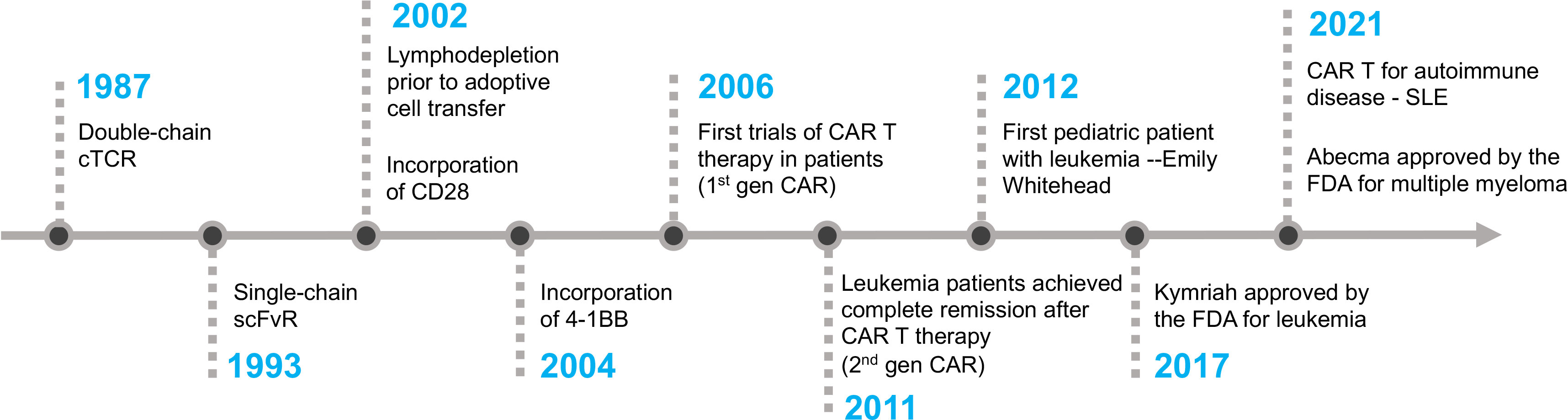
Frontiers From bench to bedside: the history and progress of CAR T

FDA and CDC salaries 2008 : U S Departmnet of Health and Human

FDA aprobă casimersen pentru distrofia musculară Duchenne o nouă
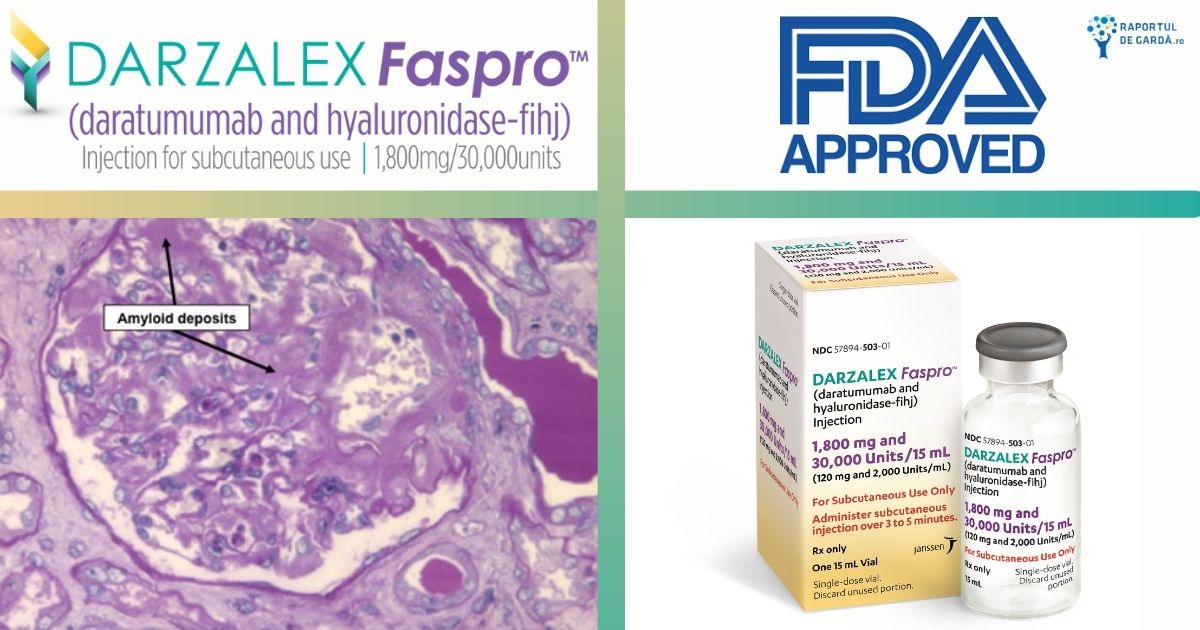
Articole despre Hematologie

FDA and CDC salaries 2008 : U S Departmnet of Health and Human
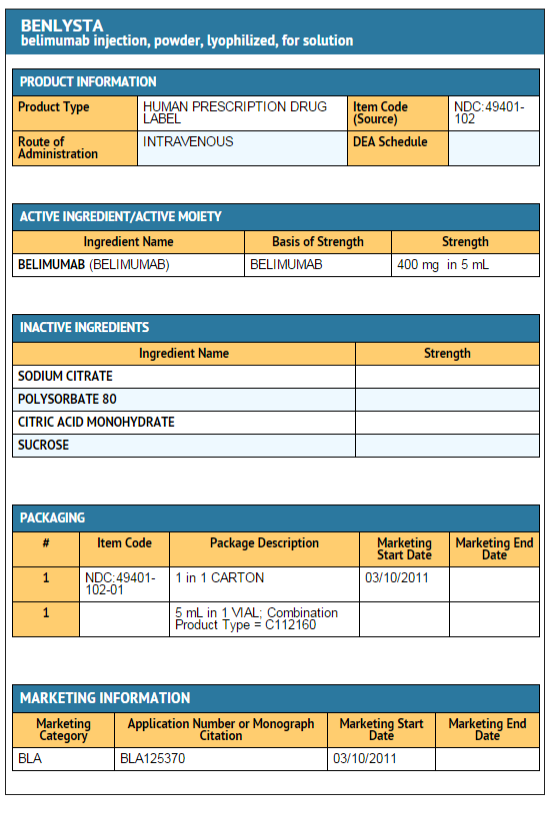
Belimumab wikidoc