Fda Protocol Template
Here are some of the images for Fda Protocol Template that we found in our website database.

Free FDA Clinical Protocol Template to Edit Online
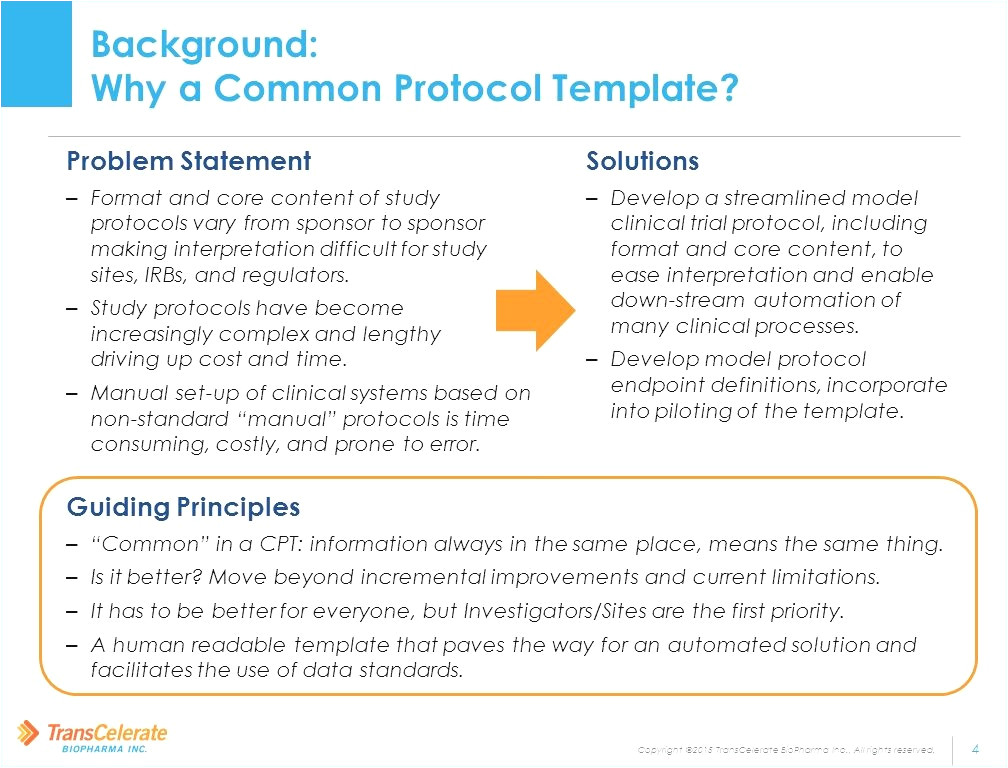
Fda Protocol Template

Fda Protocol Template

Fda Protocol Template

Sales Best Quote Practices Protocol Template in Word PDF Google Docs

Marketing Affiliate Protocol Handbook Template in Word PDF Google

Free Test Protocol Template to Edit Online

Treatment Protocol Template Edit Online Download Example Template net

NIH FDA Clinical Trial Protocol Template medicine okstate Doc
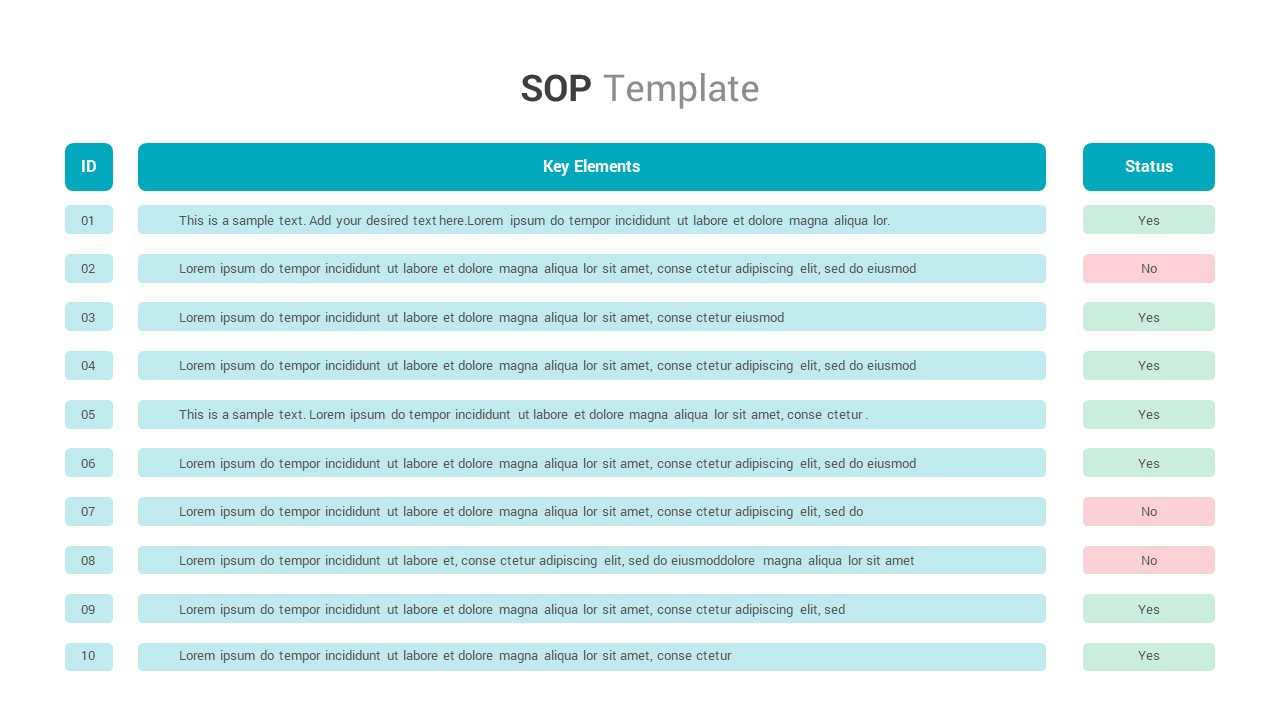
Standard Operating Protocol Template SlideBazaar
Standard Operating Protocol Template SlideBazaar

Standard Operating Protocol Template SlideBazaar

Free Study Protocol for Clinical Research Template to Edit Online
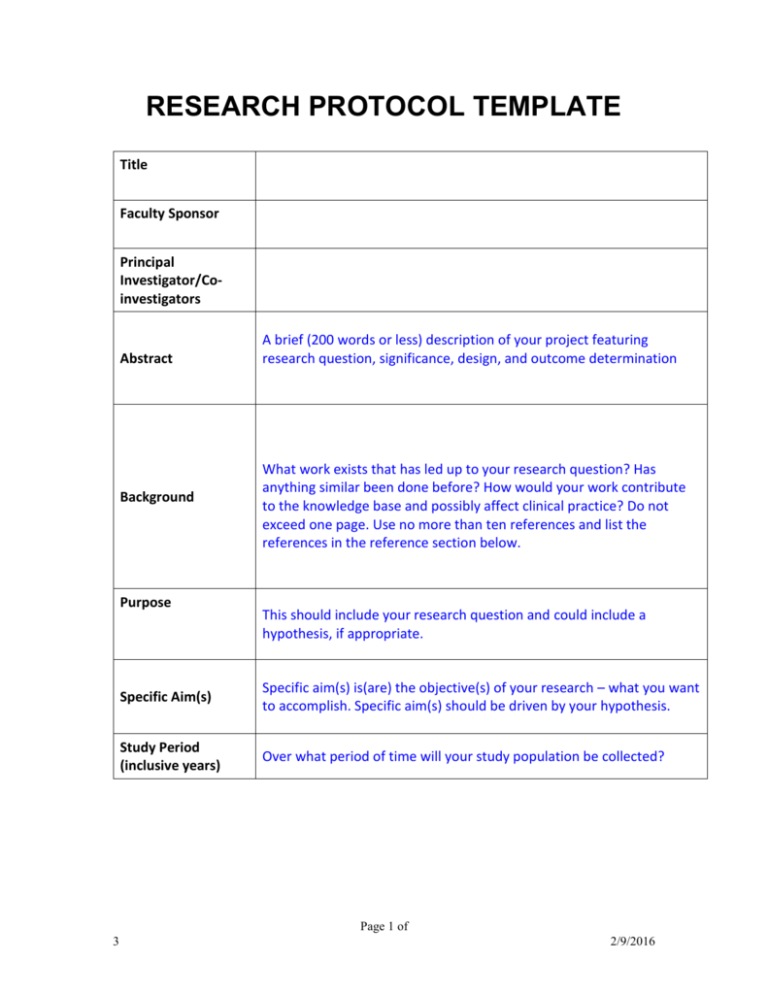
Research Protocol Template for Medical Studies

Clinical Study Protocol (CSP) Template Clinical Study Templates

Word Protocol Template

FDA and NIH finalize clinical trial protocol template Health Law Pulse

Social Media Customer Service Advertising Protocol Template in Word

Sales Custom Quote Protocol Template

Protocol Template Word
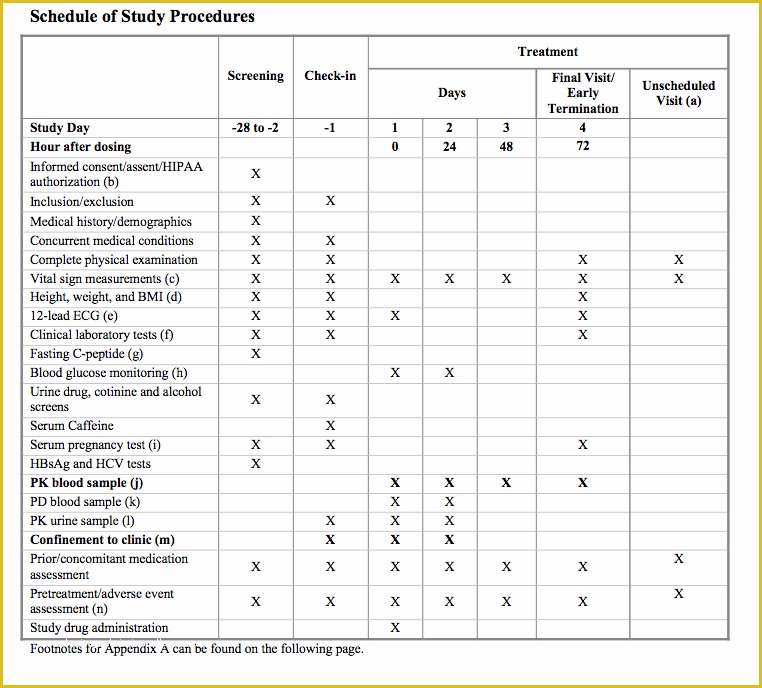
Fda Sop Template
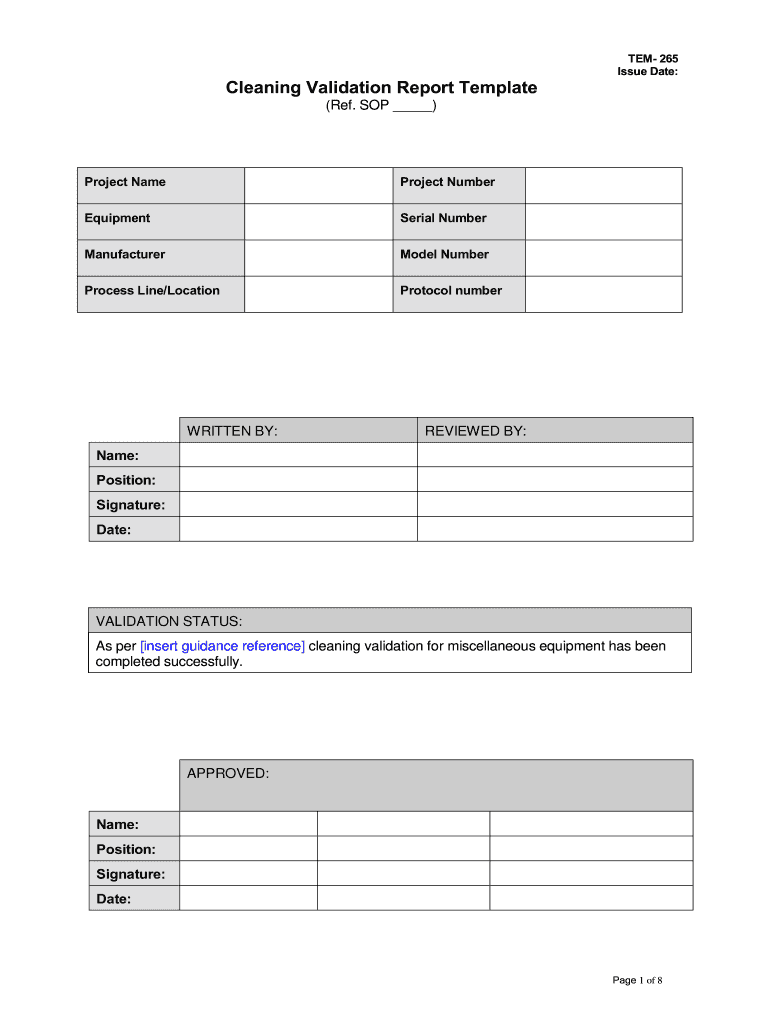
Cleaning Validation Protocol Template
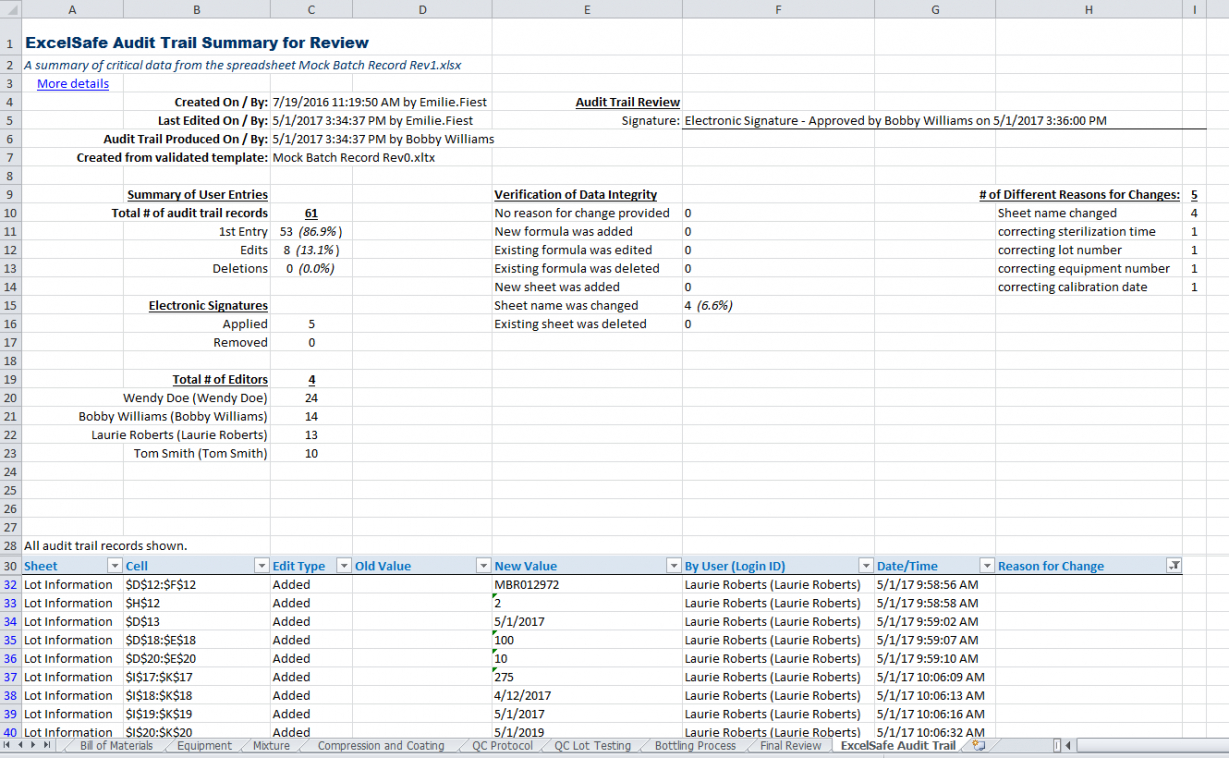
Excel Spreadsheet Validation Protocol Template db excel com
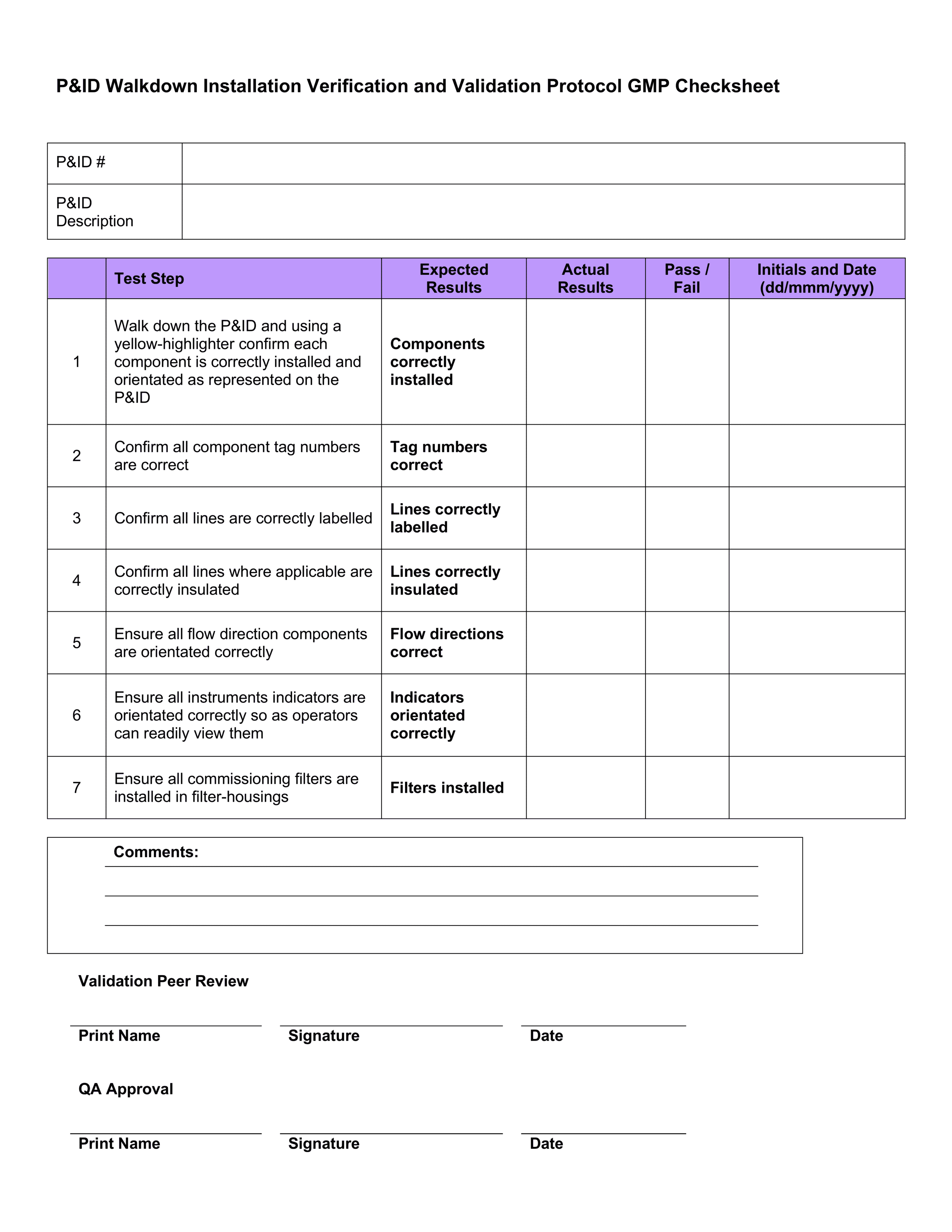
Validation Protocol Template
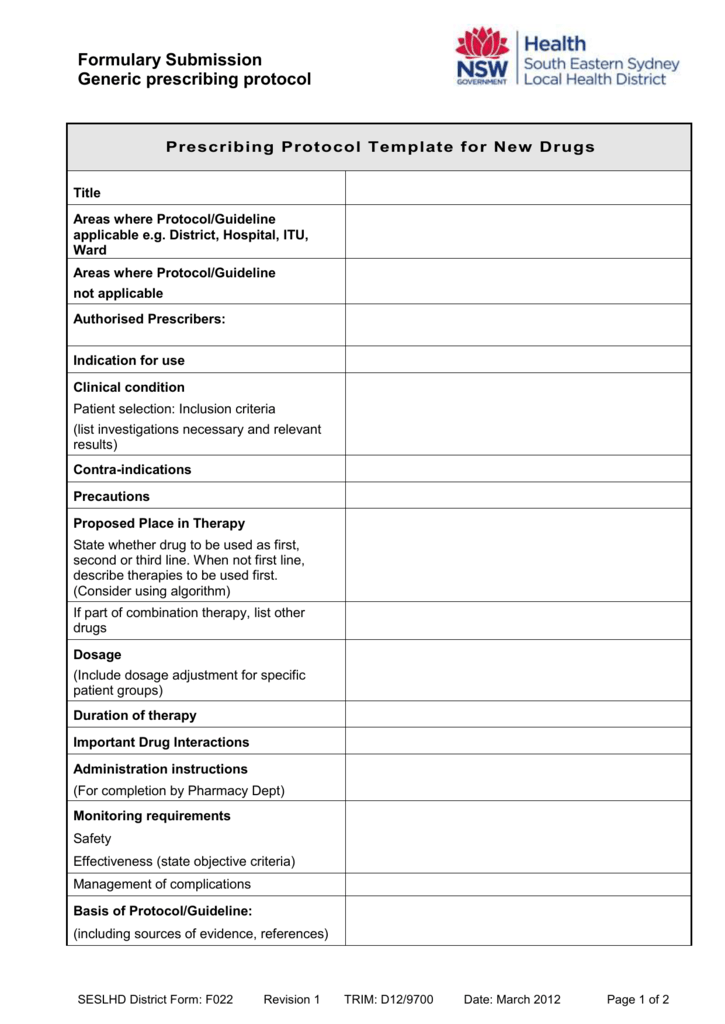
Esi Protocol Template

Medical Protocol Template

Fillable Online NIH FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol

NIH FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol Template PREFACE
Protocol: A beautiful starting point for your next API documentation

NIH FDA Phase 2 and 3 Clinical Trial Protocol Template: A Course Hero

Transcelerate Protocol Template

Temperature Mapping Protocol Template prntbl concejomunicipaldechinu

Fillable Online accessdata fda Order Set / Protocol Template U S

Sample Protocol Template

Installation Qualification (IQ) Protocol Template (Medical Device
Common Protocol Template (CPT) Initiative Collaboration with FDA and

instructions for clinical research protocol template Doc Template

Protocol Deviations in Clinical Trials: FDA Draft Guidance
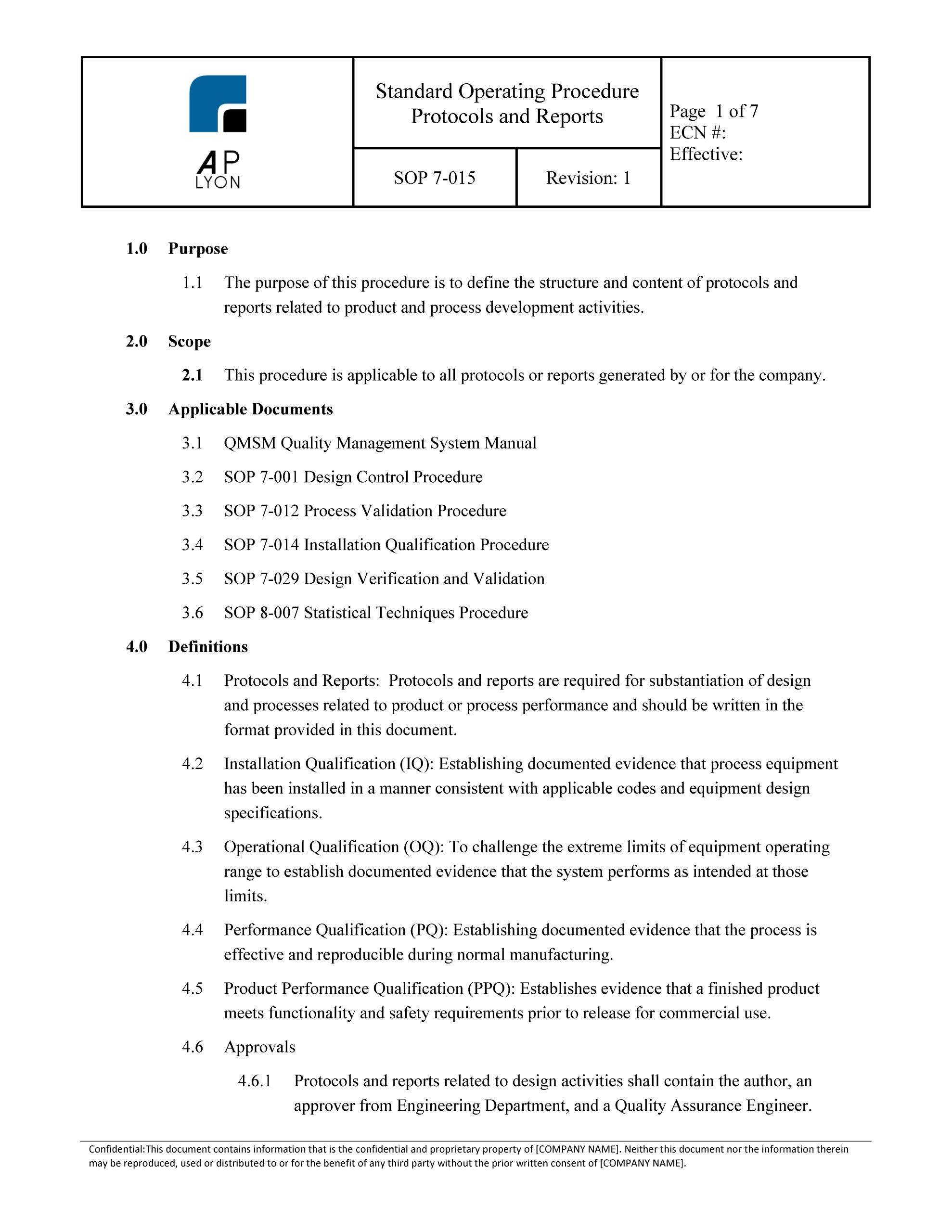
Equipment Qualification Protocol Template at Thelma Robertson blog

Free Project Declaration Template to Edit Online

Computer System Validation Protocol Template Web according to both

Computer System Validation Protocol Template Web according to both

Regulation and Standard Library The FDA Group

New FDA Protocol Deviation Guidance: Planning for the Things That Don t

New FDA Protocol Deviation Guidance: Planning for the Things That Don t

New FDA Protocol Deviation Guidance: Planning for the Things That Don t

Pilot Test Agreement Template Sfiveband com
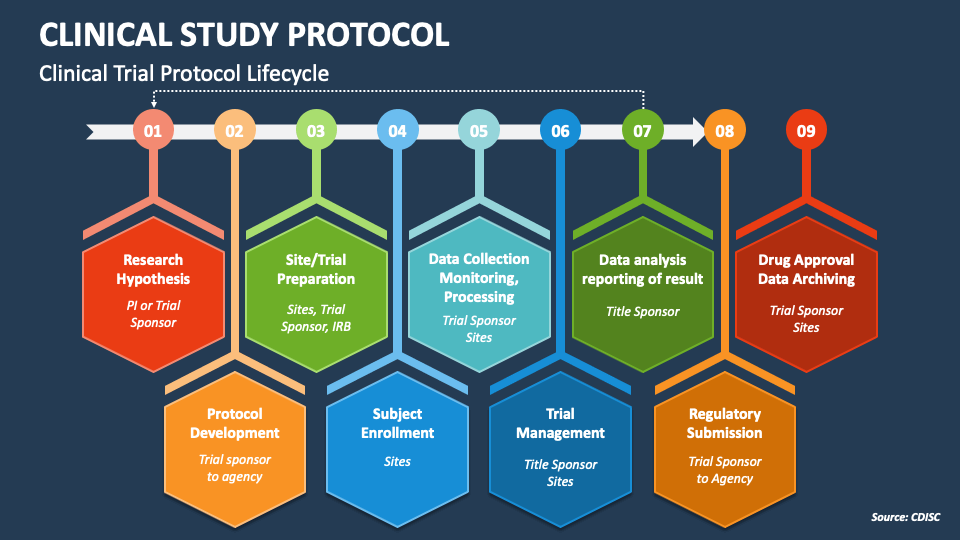
Clinical Study Protocol PowerPoint and Google Slides Template PPT Slides
Radio Advertising Template prntbl concejomunicipaldechinu gov co

Radio Advertising Template prntbl concejomunicipaldechinu gov co
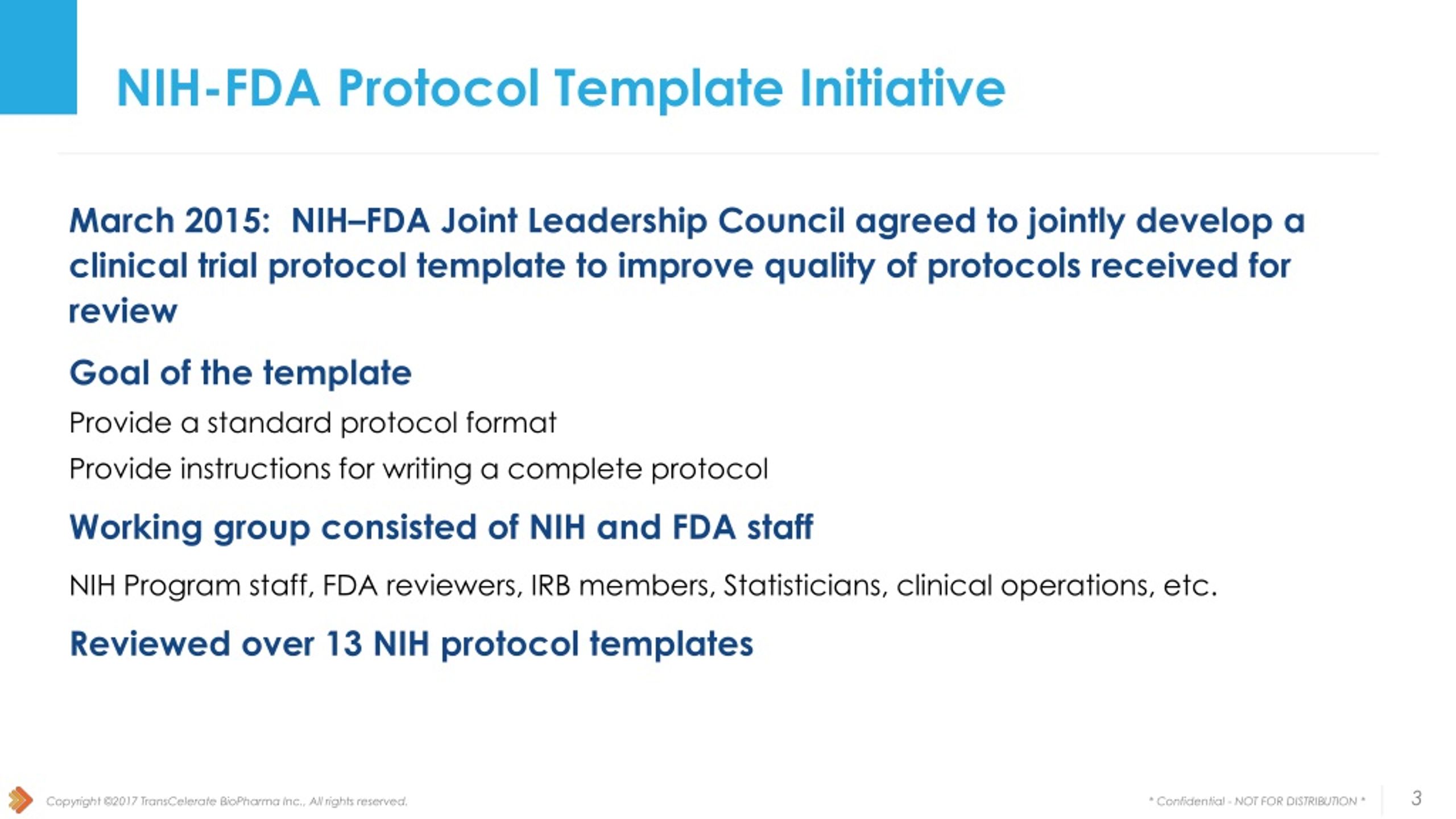
PPT Collaboration with FDA and NIH PowerPoint Presentation free
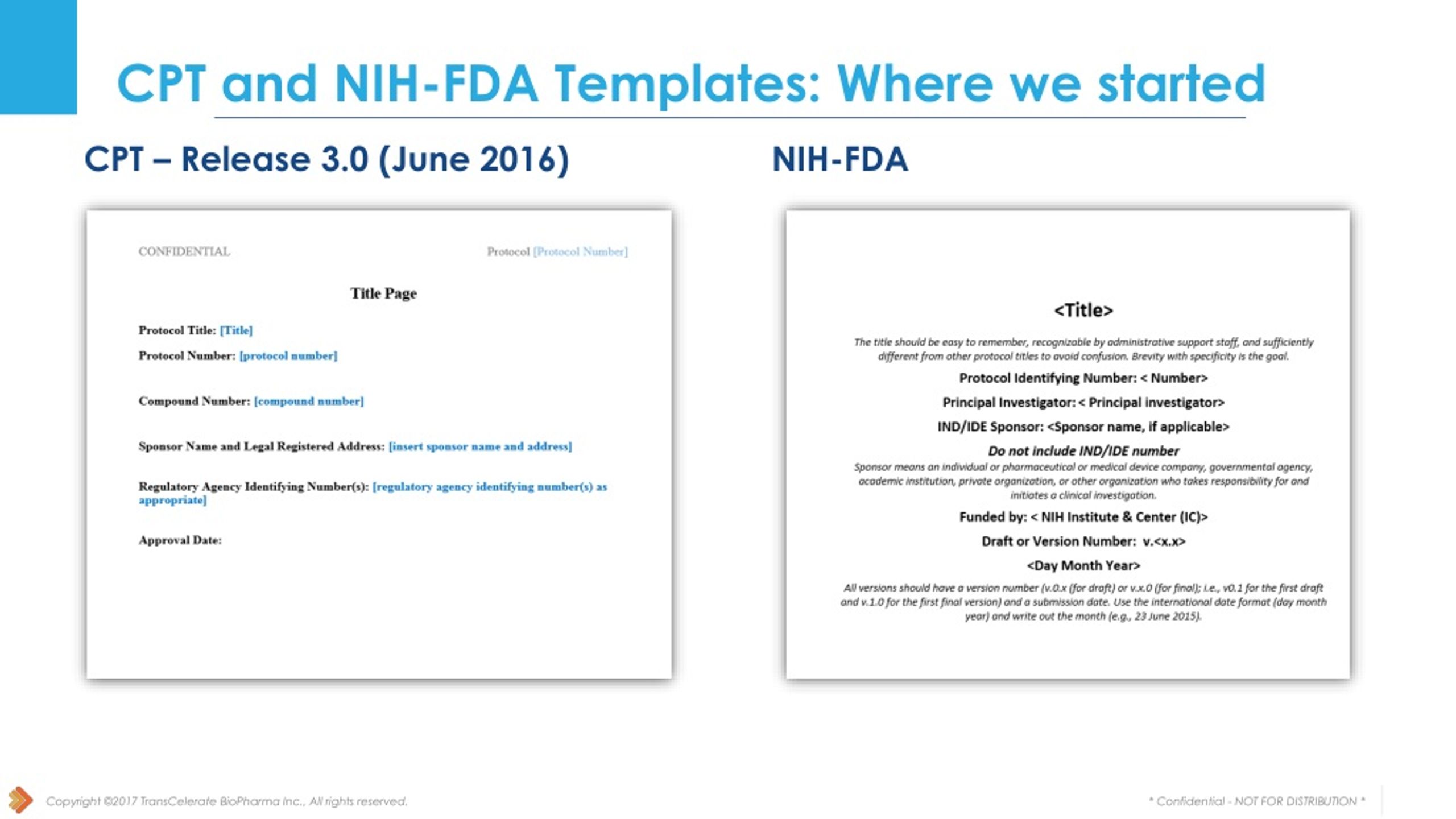
PPT Collaboration with FDA and NIH PowerPoint Presentation free
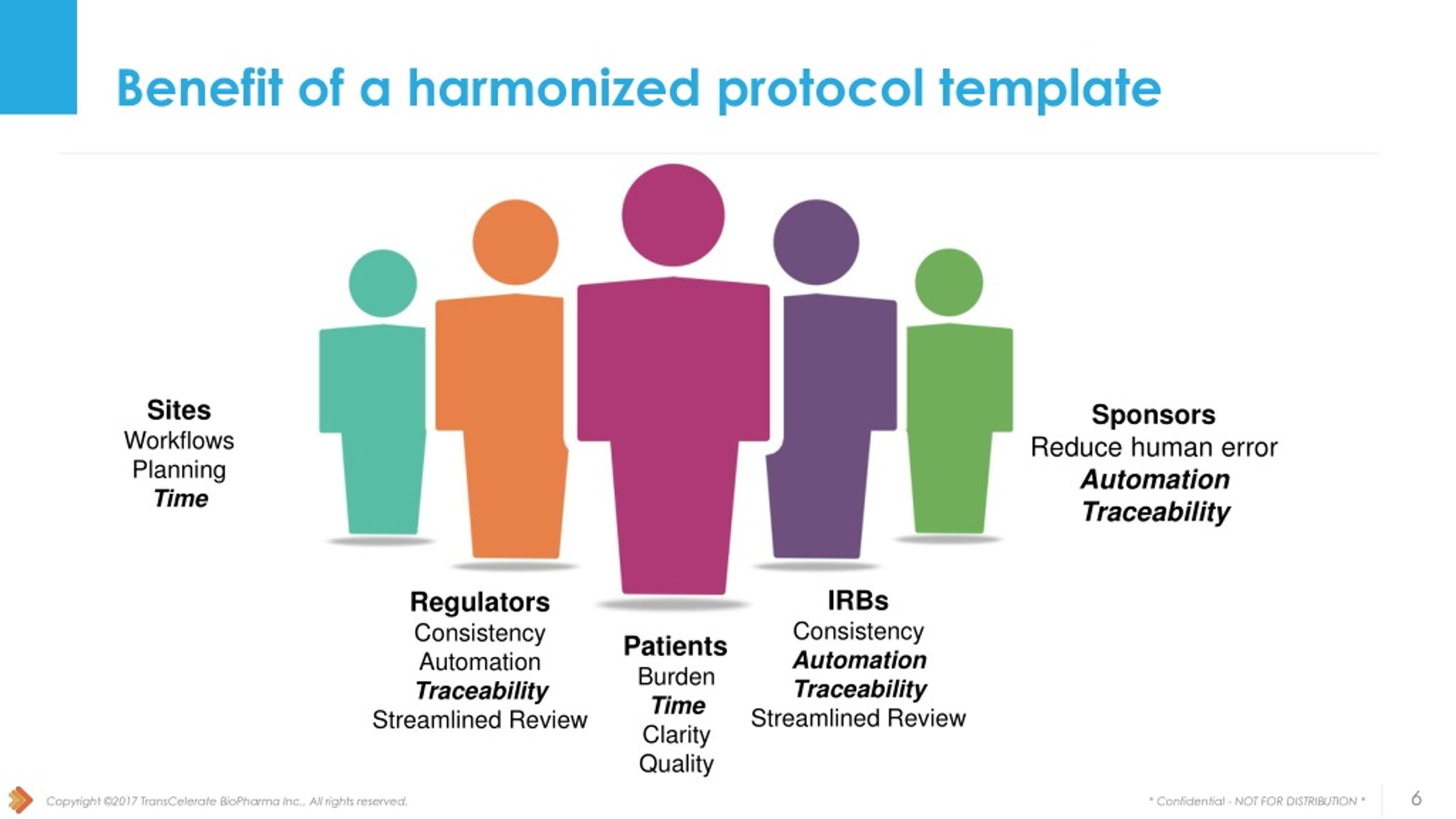
PPT Collaboration with FDA and NIH PowerPoint Presentation free

PPT Collaboration with FDA and NIH PowerPoint Presentation free

Instructions for Organ Dysfunction Studies: Pharmacokinetics Course Hero

Hold Time Study Protocol for Pharmaceutical Products Pharma Digests

Shelf Life Procedure

Fillable Online fda Warning letter Food and Drug Administration fda

Participant timeline GUIDANCE FOR CLINICAL TRIAL PROTOCOLS