Gamp 5 Certification
Here are some of the images for Gamp 5 Certification that we found in our website database.

GAMP 5 Masterclass Gaurav Dakshini

GAMP5 GUIDELINES PDF
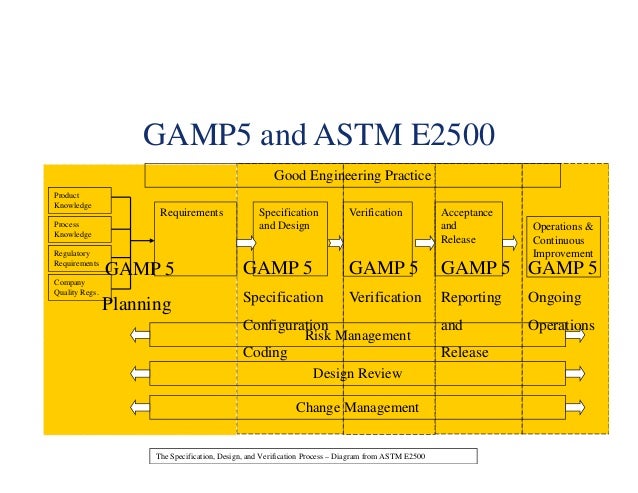
GAMP5 GUIDELINES PDF

Gamp 5 guidelines bopqestation

Gamp 5 PDFCOFFEE COM
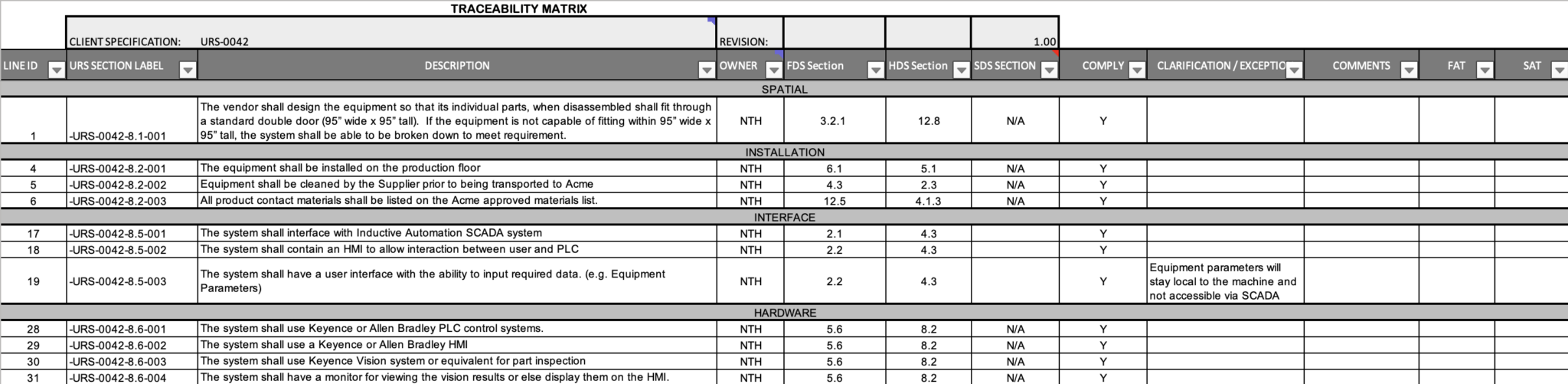
Simplifying GAMP Validation: Automation NTH Validation Support
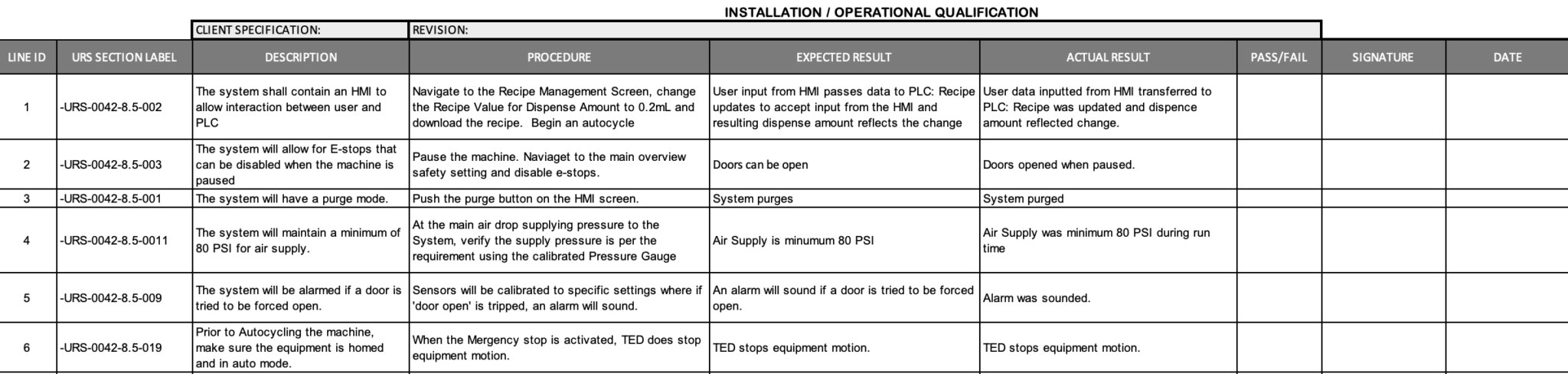
Simplifying GAMP Validation: Automation NTH Validation Support
GAMP 5 vs GAMP 5 2nd Edition Guideline Scilife

GAMP® 5 Second Edition CSV/GxP Validation Training and Audits
GAMP 5 and GAMP 5 2nd Edition: What are the main differences? Scilife
GAMP 5 and GAMP 5 2nd Edition: What are the main differences? Scilife

What is GAMP 5 Guideline? Checklist for GAMP 5 compliance Zamann
GAMP 5 Computer System Validation (CSV) Data Integrity etc

GAMP®5 2nd Edition released ECA Academy

Pharmaceutical Facility Design Gamp 5 Certification 879x720 PNG

Compliance with GAMP 5 guidance: A checklist Tricentis

My view on the GAMP 5 Guide 2nd Edition

GAMP 5 Guidelines for Computerized System Validation

What is GAMP 5 Guideline ? Checklist for GAMP 5 compliance
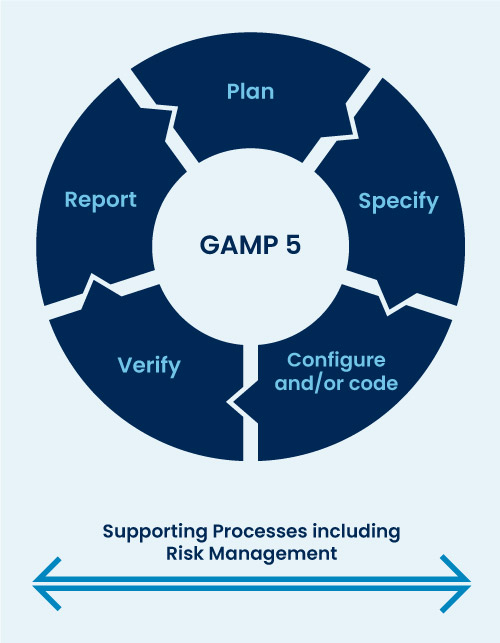
Agile GAMP5 Validation for Medical Device Software MakroCare

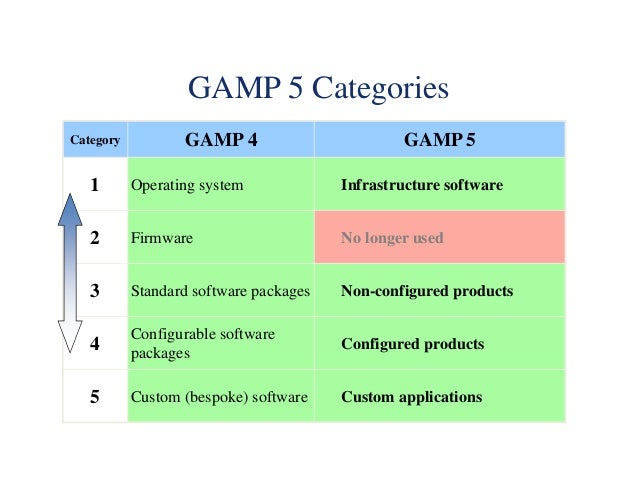
Decoding GAMP 5: Navigating Software Categories for Compliance Success

V Model as per GAMP 5 Guidelines S M Abu Baser

10 Key Changes in the GAMP 5 Second Edition

FMEA And GAMP 5: Risk Management Tools
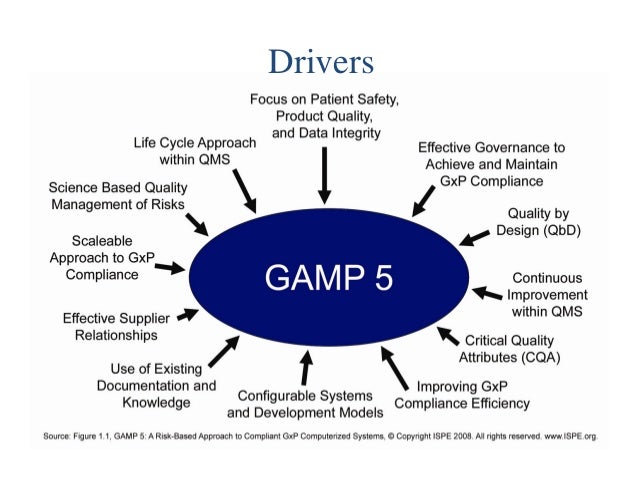
ISPE GAMP5 second edition: What s new?
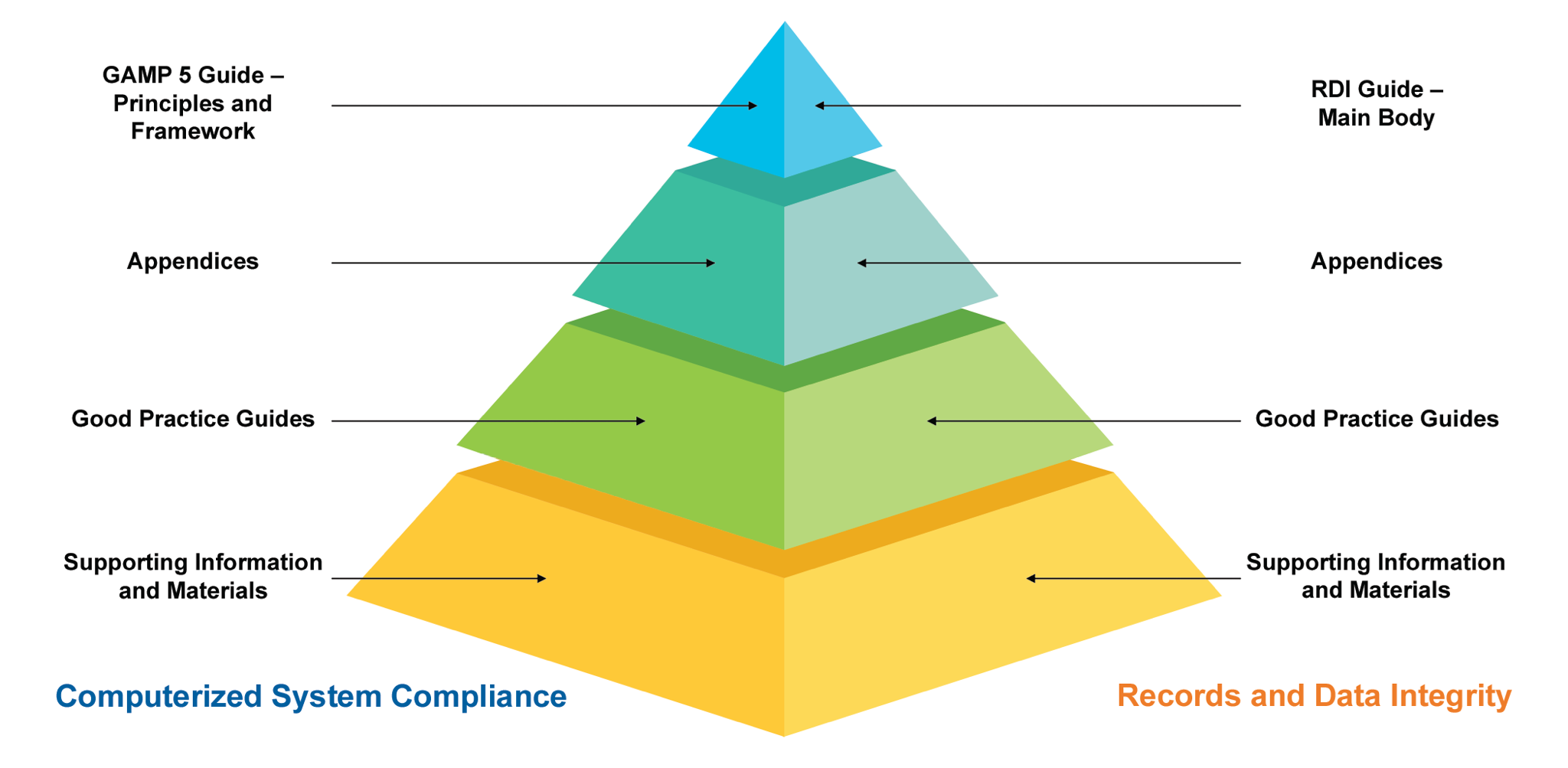
Overview of Computerized Systems Compliance Using the GAMP® 5 Guide
Overview of Computerized Systems Compliance Using the GAMP® 5 Guide

The Main Differences between 1st and 2nd Editions of GAMP® 5

What is the GAMP 5 V model in Computerized System Validation? QbD Group

Computer Validation The GAMP®5 Approach ECA Academy
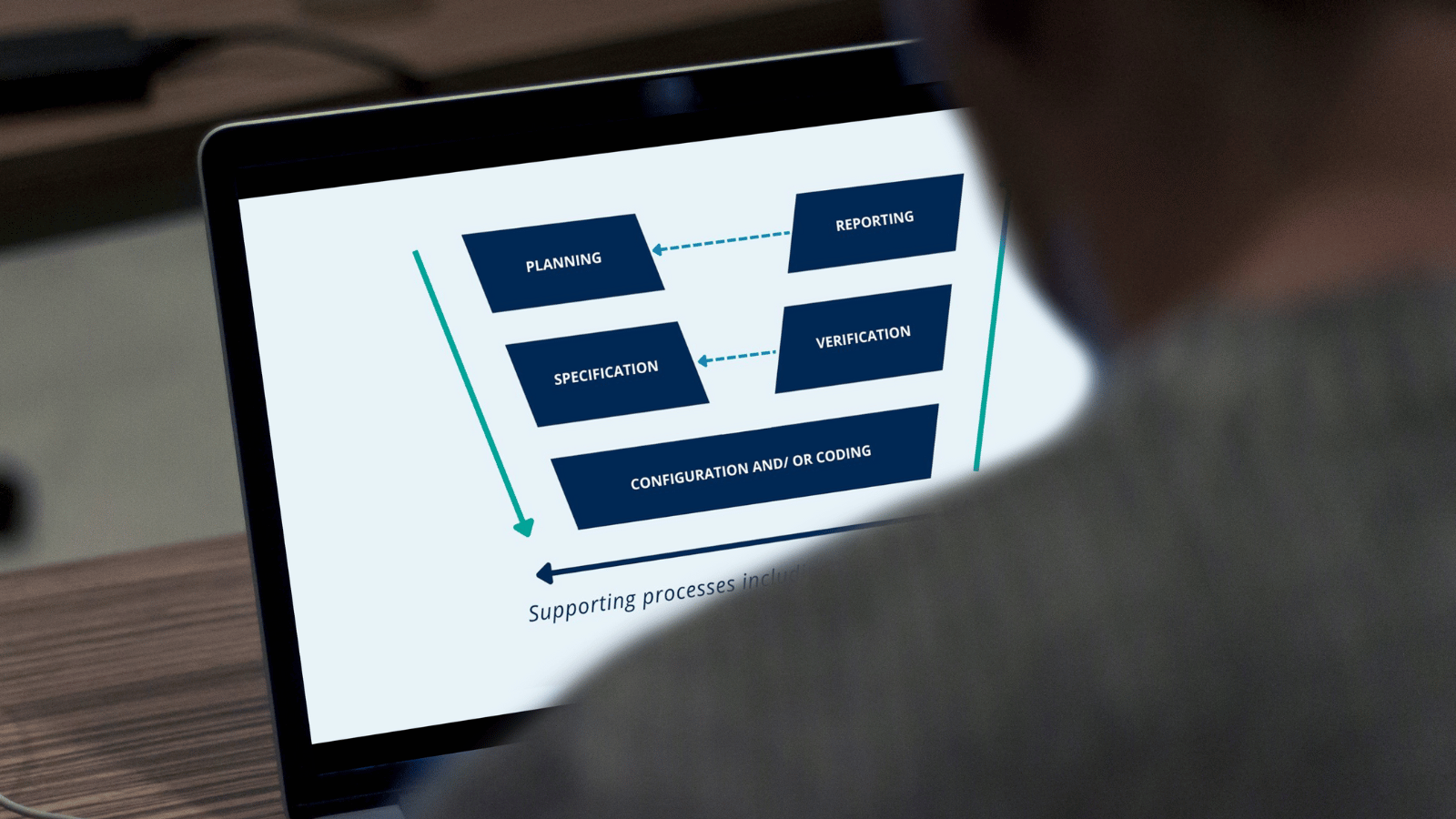
What is the GAMP 5 V model in Computerized System Validation? QbD Group

Laetitia Tardif on LinkedIn: #ISPE #GAMP #feedback

Jenny Wrede on LinkedIn: GAMP 5 Guide 2nd Edition

The importance of using the GAMP®5 guidelines for your QMS Feed4ward

A brief on GAMP 5 Categories V Model and 21 CFR Part 11 EU Annex 11

Principales cambios de la Guía GAMP 5 2ª edición

Cambio de la Guía GAMP 5 2ª edición Pharmatech
IQS e ISPE Iberia coordinan la presentación de la segunda edición de la

GAMP 5の第2版がリリースされました! 青柳 伸宏
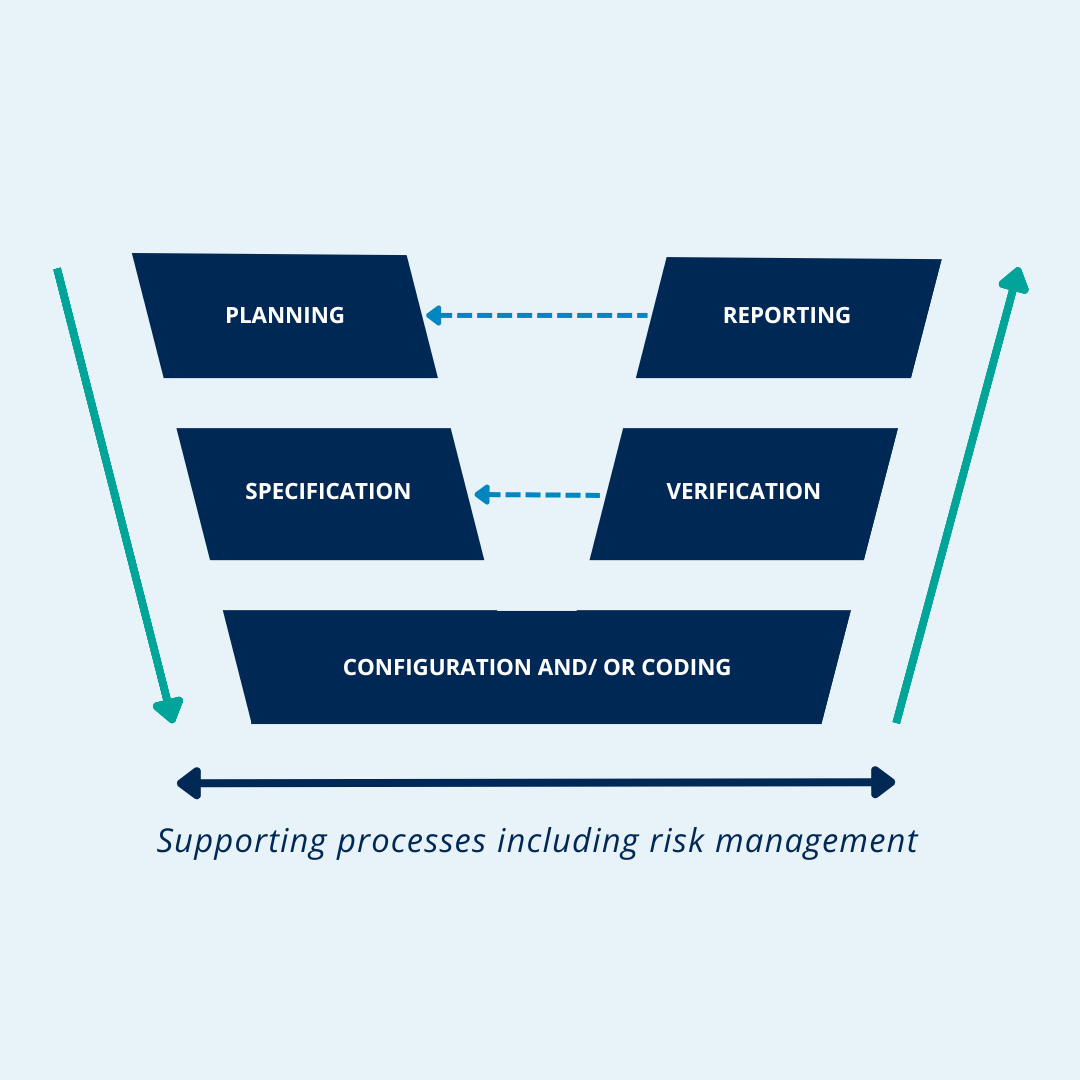
¿Qué es el V model de la guía GAMP 5 en la validación de sistemas

【GAMP 5対応】CSV対応ポリシー イーコンプレス

GAMP 5 Quality Risk (traducido) Reimpreso de Tabla A GAMP 5

GAMP 5 2nd Edition Chapter 2 YouTube

Comissioning qualification vol 5 (2nd eddition) PDF

Selecting the Right GAMP® 5 Software Category for Your Environmental

security certificate GAMP 5

Ray Randy are at the Seven Lakes Oilfield Services Facebook

Certificate for solidworks taiwanplm
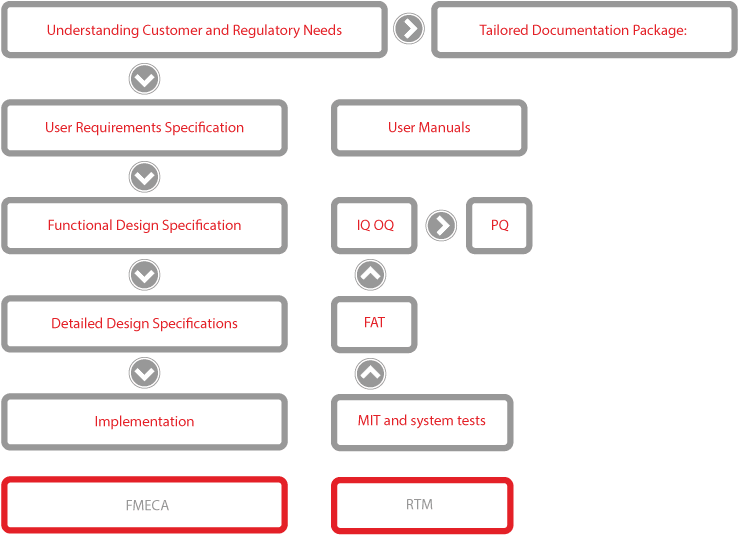
Validation

CSV: Computer System Validation Basic Concepts GAMP®5 Training

GxP Assessment docx

GxP Dictionary

CSV vs CSA: What Are the Main Differences?

Curso Int Validación de sistemas computarizados según metodología GAMP 5
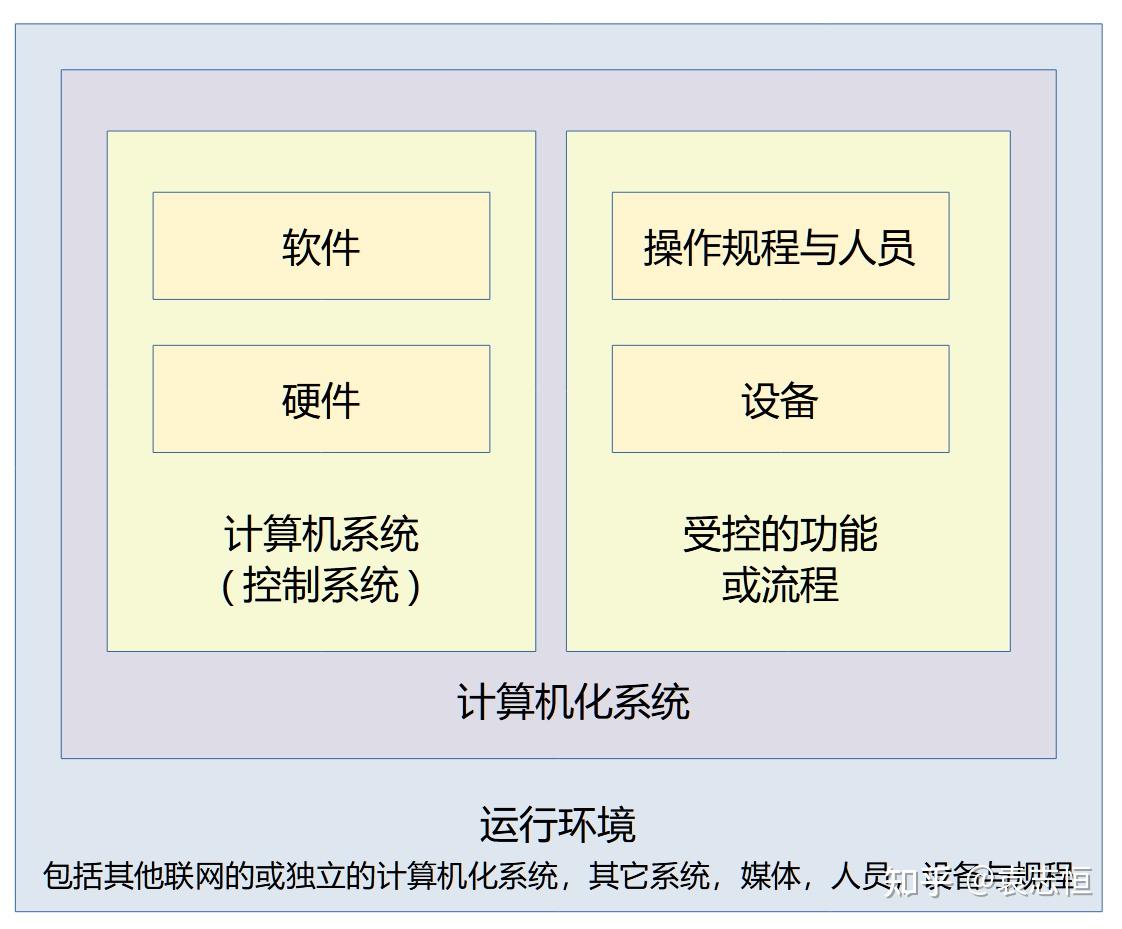
GAMP 5 Guide 2nd Edition 阅读笔记 计算机化系统 知乎
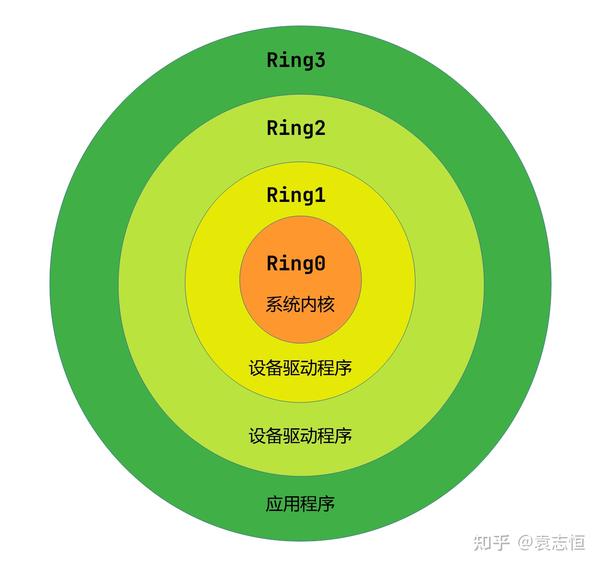
GAMP 5 Guide 2nd Edition 阅读笔记 系统分类 知乎

GAMP®5第二版回顾 知乎

GAMP®5第二版回顾 知乎

Yo asi 27001 certificado Insignia o información seguridad

新出行 GAMP 评测 LOGO 正式发布! 评测 新出行