Iec 62304 Certification
Here are some of the images for Iec 62304 Certification that we found in our website database.

Using Vector Tools for Medical Software Certification According to IEC

IEC 62304 standard for medical device software development MATLAB

IEC 62304 FDA Software Validation Compliance QA Systems

IEC 62304 LDRA

IEC 62304 LDRA

IEC 62304 Certification Technical Consultants For Guidance
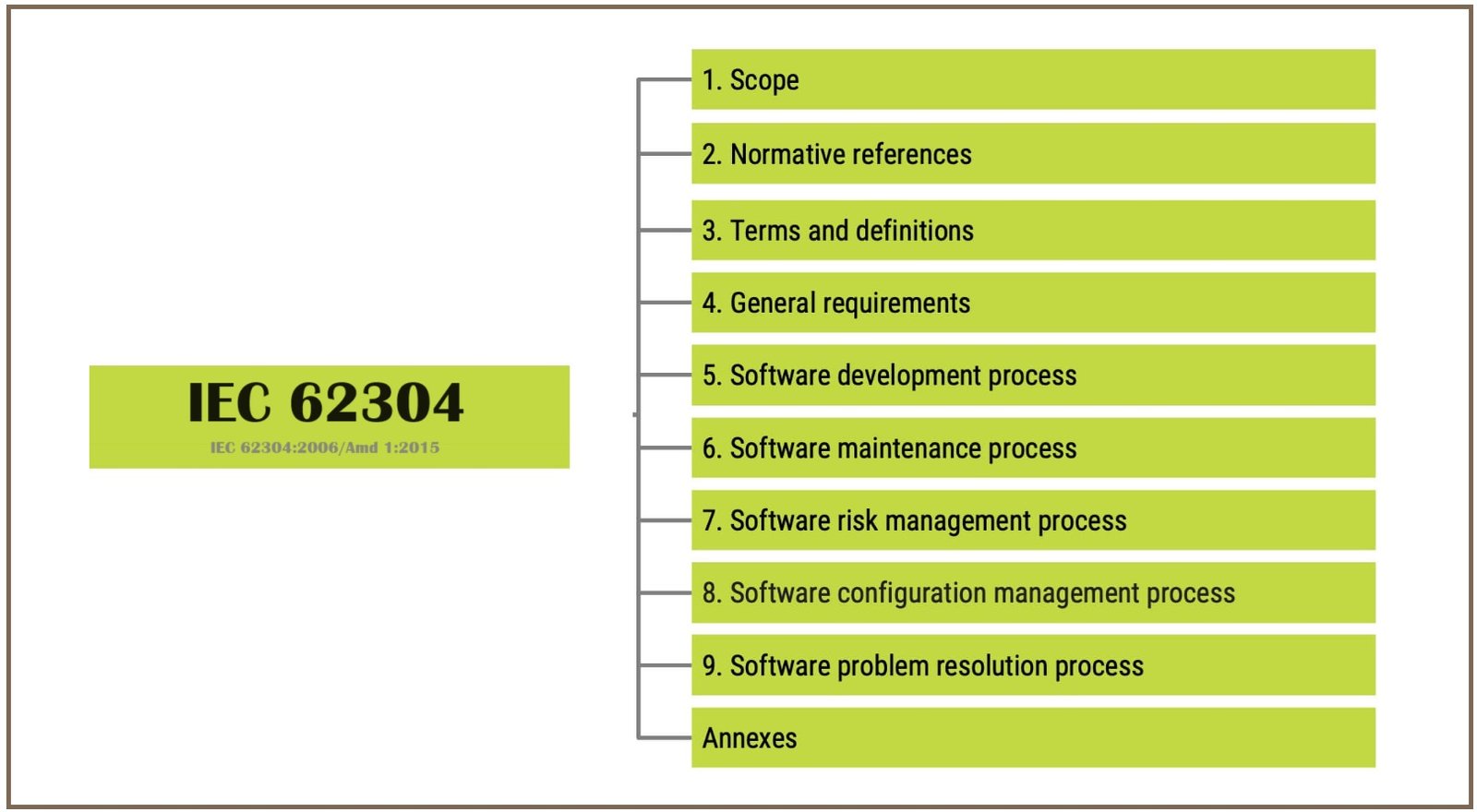
IEC 62304 Standard Implementation Software Classification

ISO 62304 Certification

IEC 62304 Visure Solutions

IEC 62304 Certification Consultants and and List of Procedures

The IEC 62304 Compliance: Medical Software Guidelines

IEC 62304 and ISO 13485 Certification Services I3CGLOBAL

IEC 62304 and ISO 13485 Certification Services I3CGLOBAL
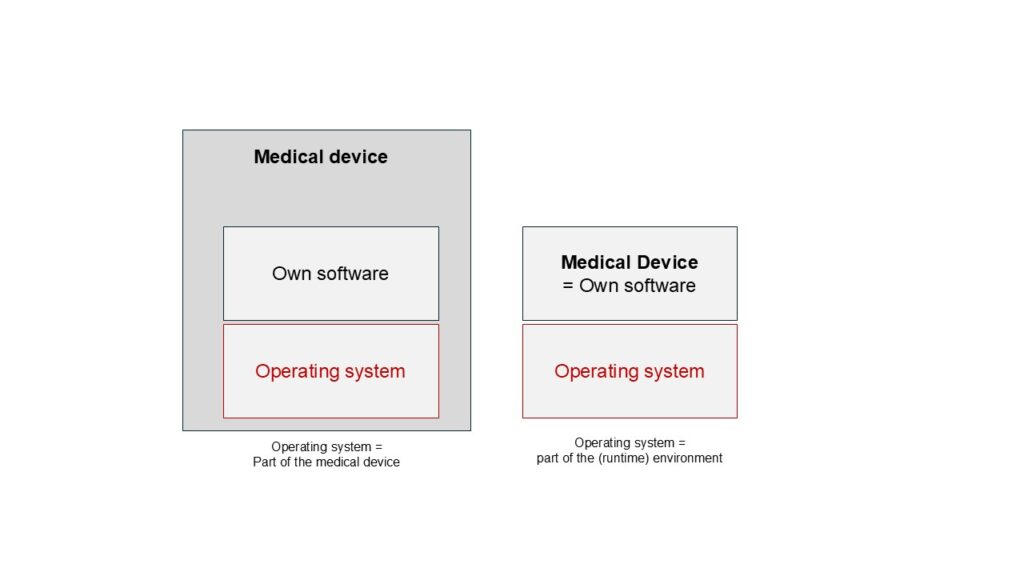
Operating system compliant with IEC 62304 and FDA?
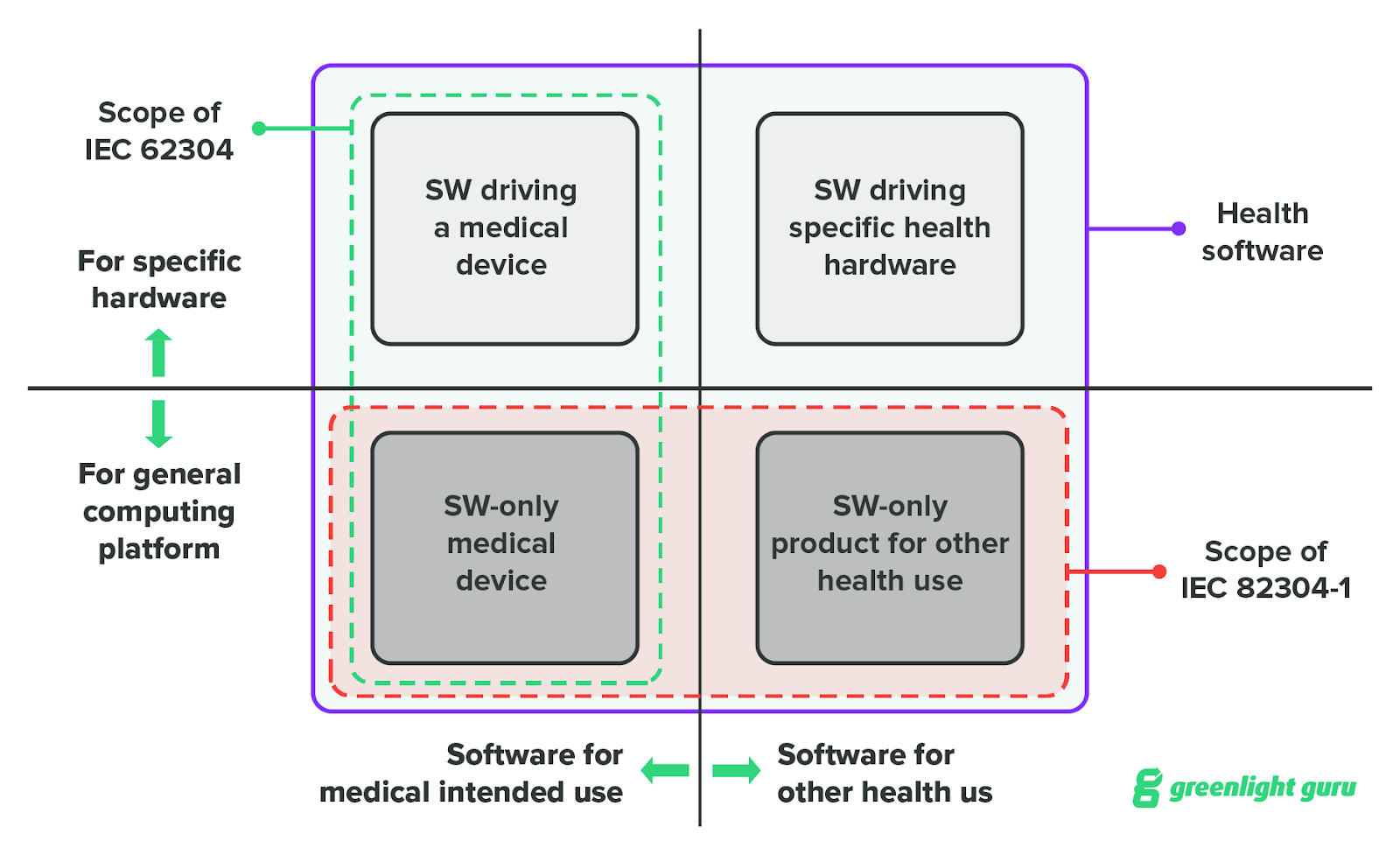
IEC 82304 vs IEC 62304: Software Standards for SaMD Explained

IEC 62304 Risk Management For Medical Devices Visure Solutions

IEC 62304 How to Overcome Challenges in the Medtech?

IEC 62304: The Complete Certification Guide Visure Solutions

Medical Devices Speaker Series 2023: Achieving IEC 62304 Certification

What is IEC 62304? Ensuring safety in medical software

Medical Devices Speaker Series 2023: Achieving IEC 62304 Certification

IEC 62304 A thorough yet simple guide

IEC 62304: Navigating the Path to Medical Device Software Compliance

Wepik Driving Success Navigating The Fdaiso Certification Pathway For

The Role of CDSCO in SaMD Certification: Understanding IEC 62304 for
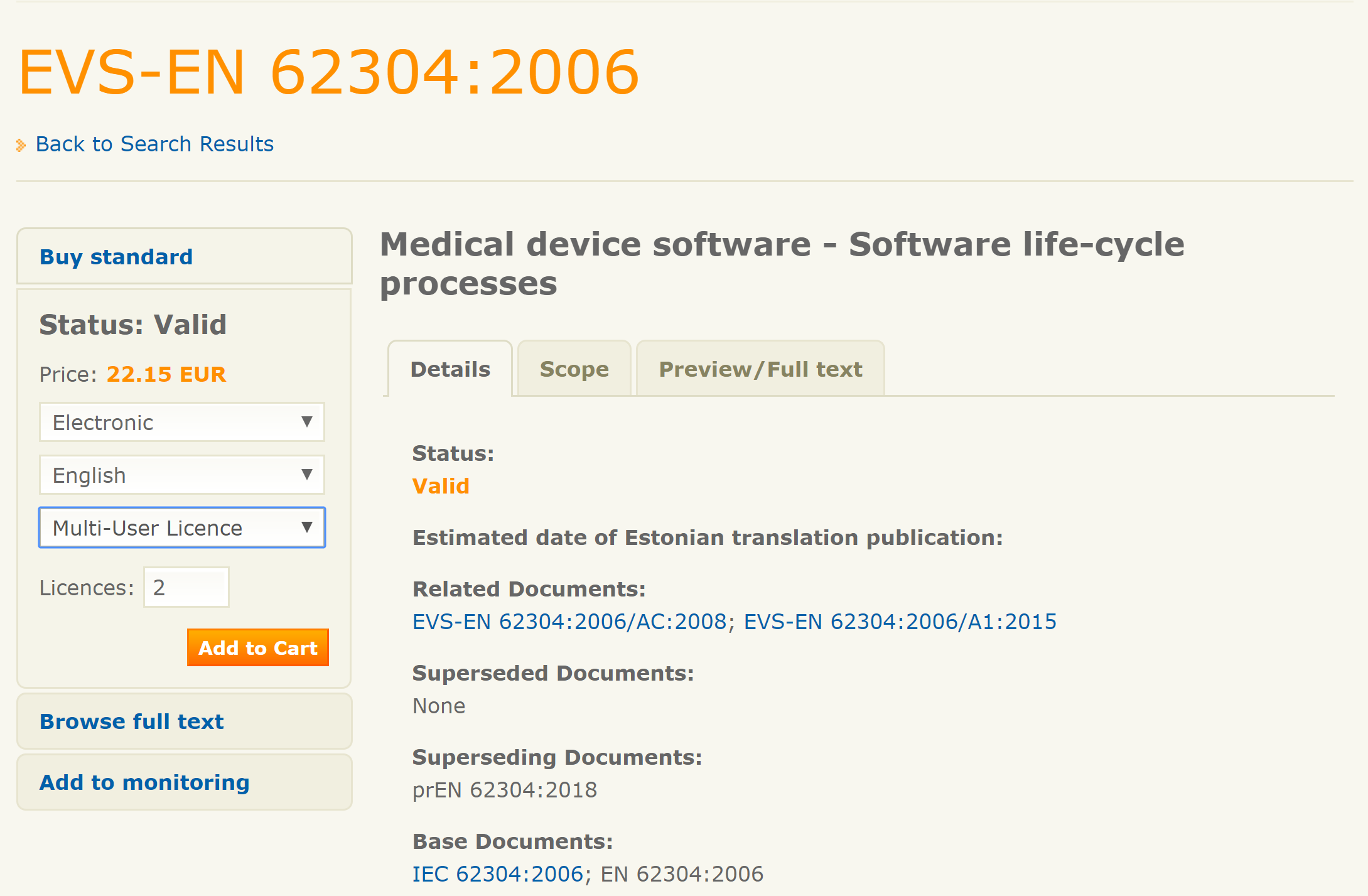
IEC 62304 2006 from evs ee website Medical Device Academy

IEC 62304:2006 Medical Device Software Software Life Cycle

Developing IEC 62304 Compliant Embedded Software for Medical Devices

What Is IEC 62304? Overview IEC 62304 Certification Compliance Tips

Download now: quot How to Achieve IEC 62304 Compliance

About Us

Design West: LDRA offers IEC 62304 and ISO 14971 certification
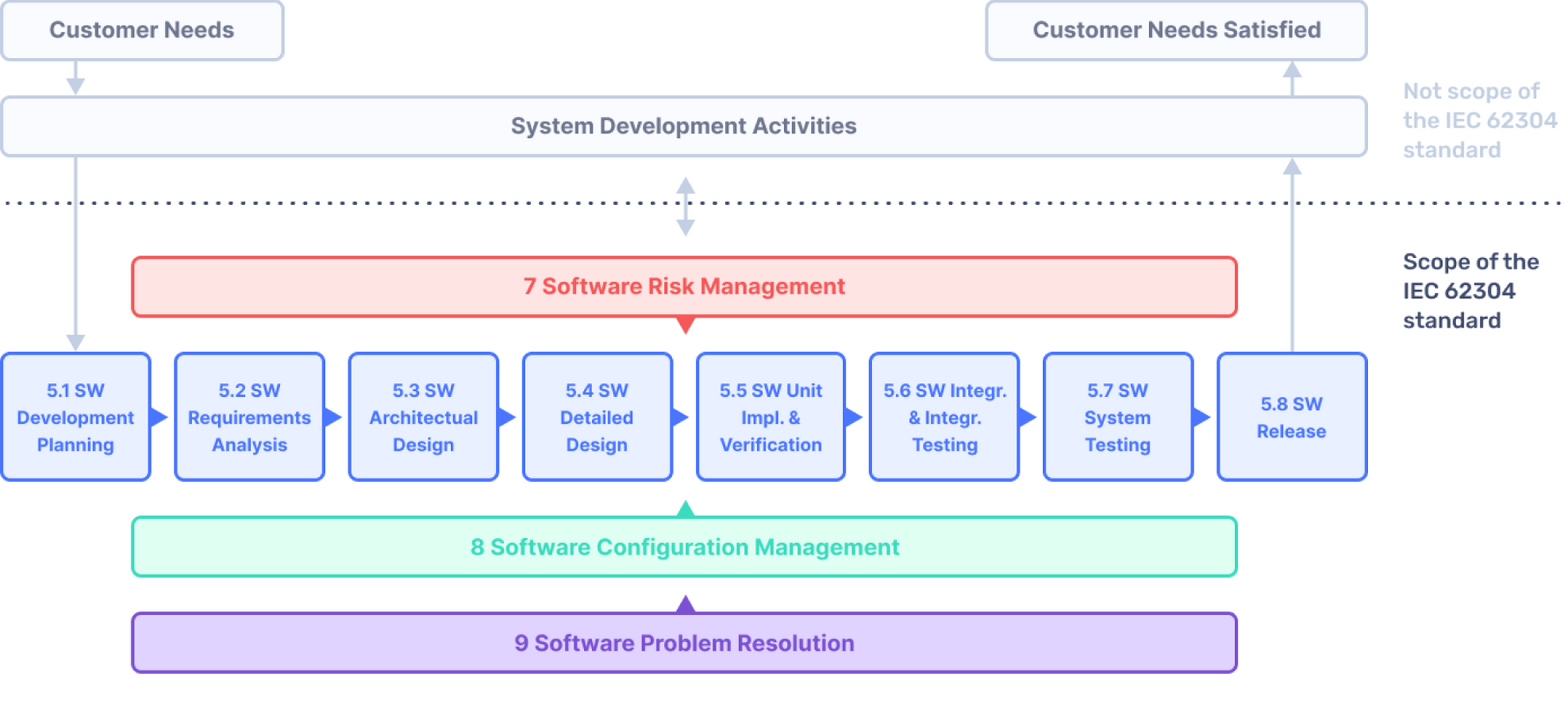
All you need to know about IEC 62304

IEC 62304 standard for medical device software What do you risk if

Design West: LDRA offers IEC 62304 and ISO 14971 certification

Khawaja Medical Technology Achieves IEC 62304 Compliance for ECG

Developing Verifying and Validating IEC 62304 Compliant Code with

Relationship of key MEDICAL DEVICE standards to IEC 62304 18

IEC 61508 IEC 62304 EN 50128 and ISO 26262 Certification Vector

IEC 62304 Medical Device Software TÜV SÜD

prEN IEC 62304:2021 Health software Software life cycle processes

IEC 82304 1 • BrightInsight

Naviguer la norme IEC 62304 pour les logiciels médicaux CLEIO

Announcing Ketryx s UL Certification to IEC 62304 ISO 13485 and ISO 14971

Stefan Brenner on LinkedIn: Developing Verifying and Validating IEC

【IEC 62304対応】 ソフトウェア開発手順書一式 イーコンプレス

【IEC 62304対応】予備ハザード分析(PHA) イーコンプレス
【IEC 62304対応】ソフトウェア構成管理計画書 イーコンプレス

【IEC 62304対応】ソフトウェア保守計画書 イーコンプレス

Operon Strategist Medical Device Consultancy CDSCO 510 k

What s the difference between IEC 61508 and 61511 and ISO 26262 and
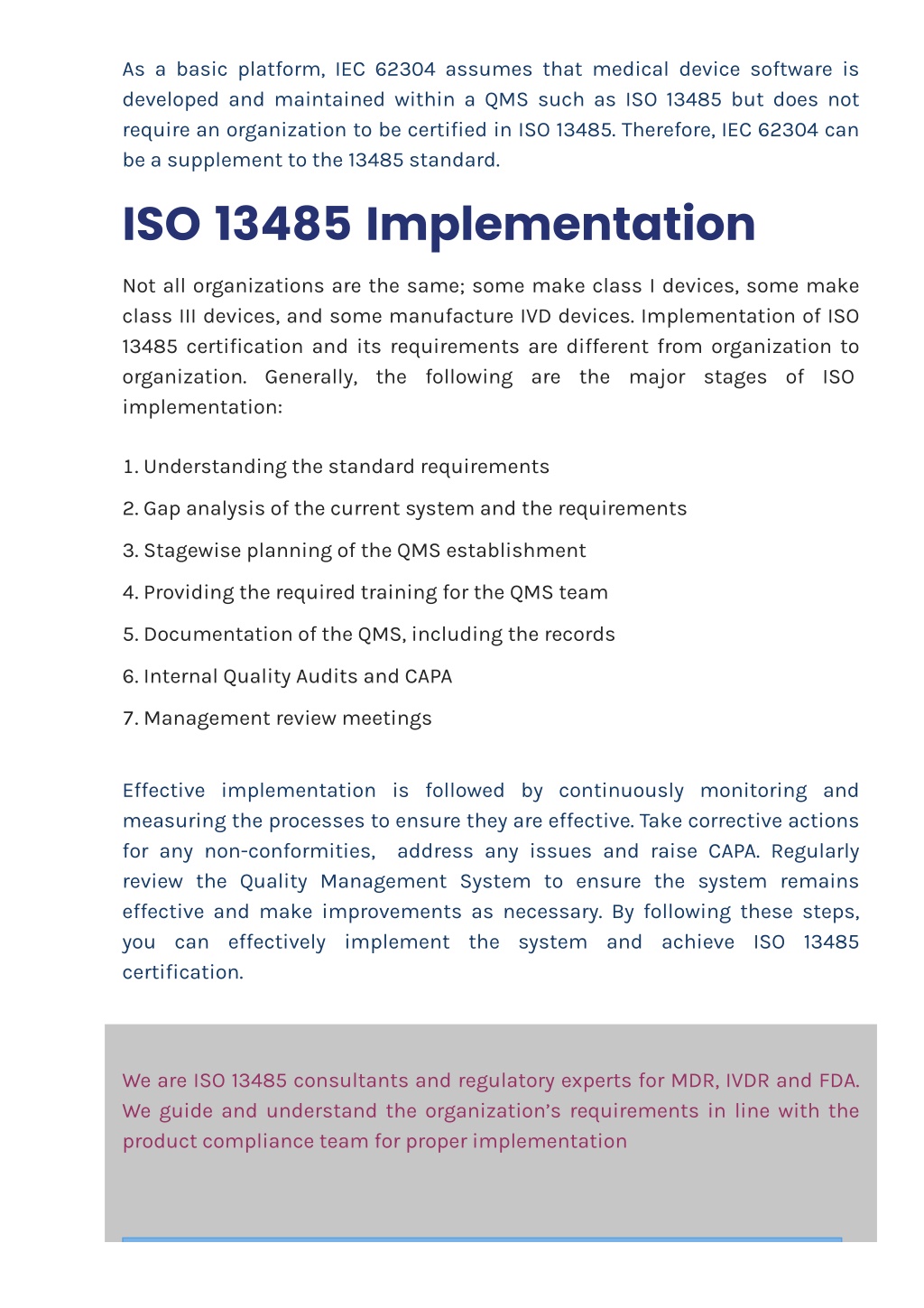
PPT ISO 13485 Certification PowerPoint Presentation free download

About ViewECG

Delivering quality: CE mark and ISO 13485 certificate Online AI
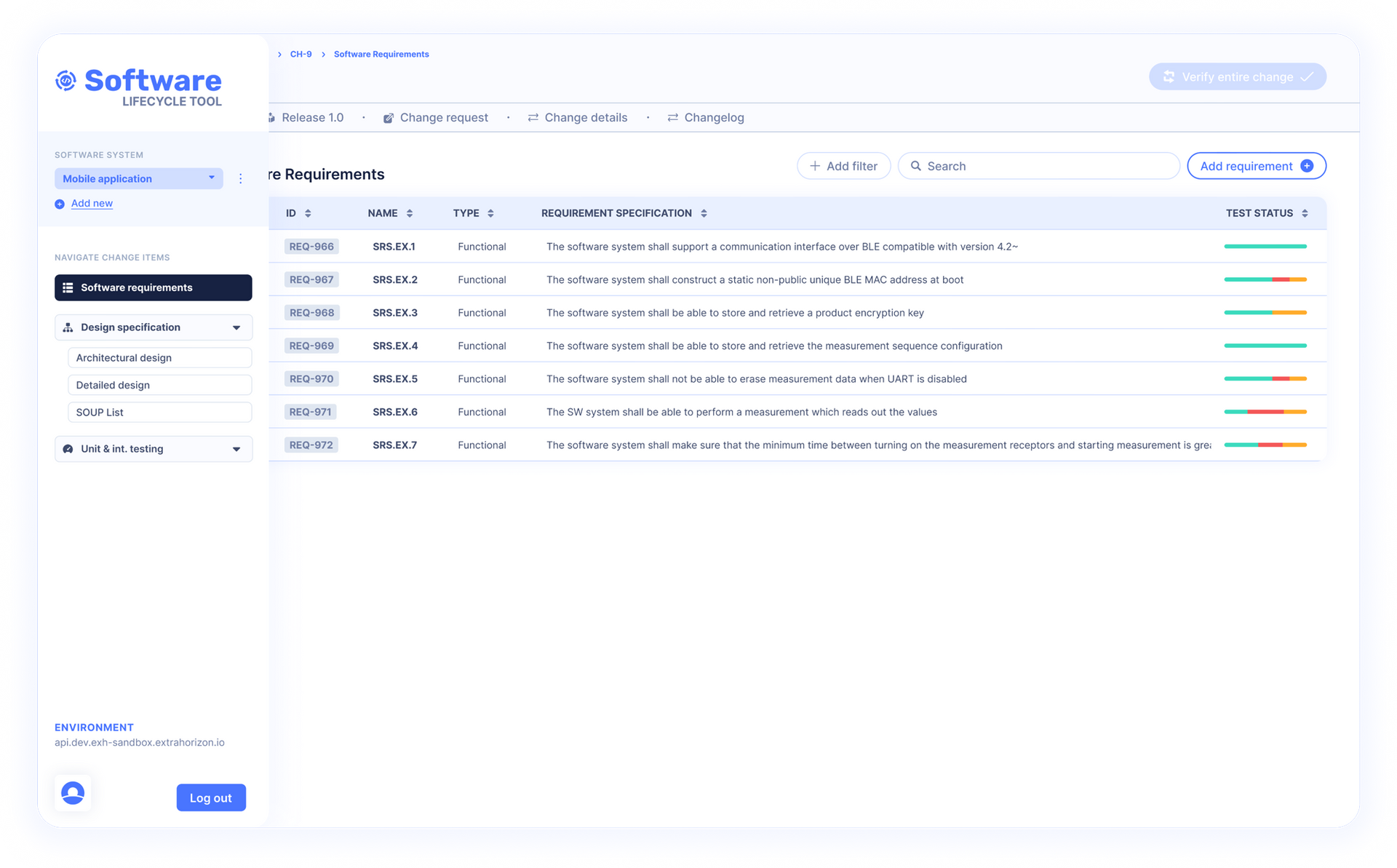
Regulatory Fast Track for Digital Medical Applications SaMD
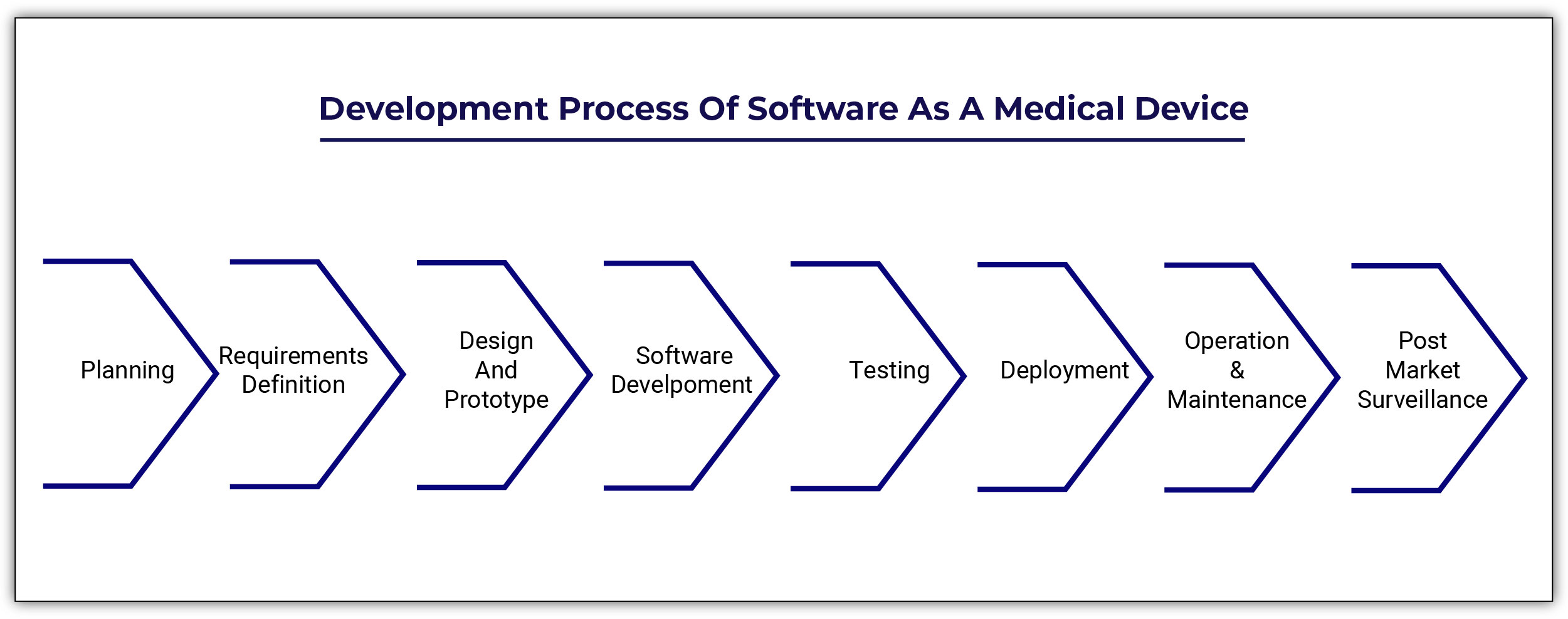
Software as a Medical Device (SaMD) IEC 62304 Certification

Software as a Medical Device (SaMD) IEC 62304 Certification

Software Safety Classification Template (IEC 62304 Medical Device

eSOL s RTOS Product Development Process Certified under IEC 62304

Embedded Software Development for Medical Devices