Medical Device Cybersecurity Certification
Here are some of the images for Medical Device Cybersecurity Certification that we found in our website database.

MEDICAL EQUIPMENT Wallpapers Wallpaper Cave

10 things you didn t know about medical residents AAMC

Medical Doctor Holding a World Gobe in Her Hands Stock Image Image of

Medical HD Wallpapers Top Free Medical HD Backgrounds WallpaperAccess

What Does a Medical Laboratory Technician Do? Carrington College
Medical Examination: Định nghĩa Cách sử dụng và Các cụm từ liên quan

All about the Pharmaceutical and Medical Device Industry Infographic
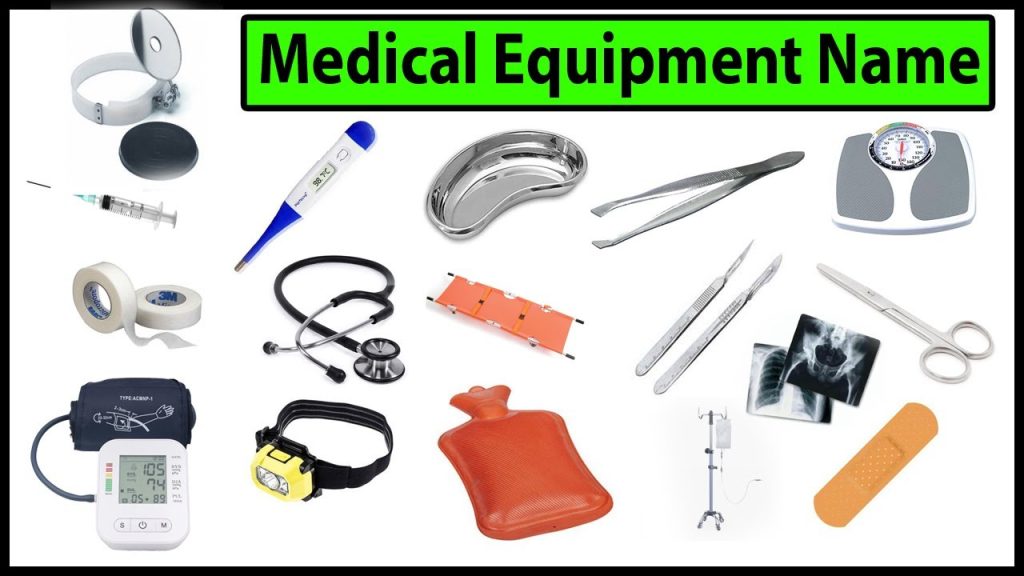
Basic Medical Equipment List Required in a Hospital Health Tips
_1662041110.png)
Decoding The Future Of Medical Technology Skill Lync Blogs

Medical Diagnosis

Benefits of AI in Healthcare

Virtual Reality Training for Healthcare: The New Tool in Medical Education

Healthcare in India Sattva Consulting

Medical Wallpaper

What is the Most Important Medical Equipment?

Discover the Latest Advances in Medical Technology PaxeraHealth

Medical Imaging Diagnostics at Bradley Briseno blog

Medical Terminology: Learning Through Practice

Medical Items Vocabulary ll 130 Medical Items Name In English With

What is Medical Terminology? Explanations Helpful Resources

Medical Terminology For Healthcare Professionals 10th Editio

Medical Supplies: Useful List of 65 Medical Equipment in English
Free Medical Infographic Design Dribbble Graphics

List Of Basic Medical Terminology

15 Major Components of a Complete Medical Record

Medical Coding: A Critical Role in Healthcare Today

Medical Terminology List Printable Pdf Medical Prefixes and Suffixes

Basics Of Medical Terminology

Health vocabulary health and healthcare in english Artofit

Medical Report What Is a Medical Report? Definition Types Uses
25 Inspiring Medical Website Design Examples (With Tips) Magezon

FREE Medical Symbol Caduceus Images ClipArt Best
3d medical sign symbol concept icon 21658602 PNG
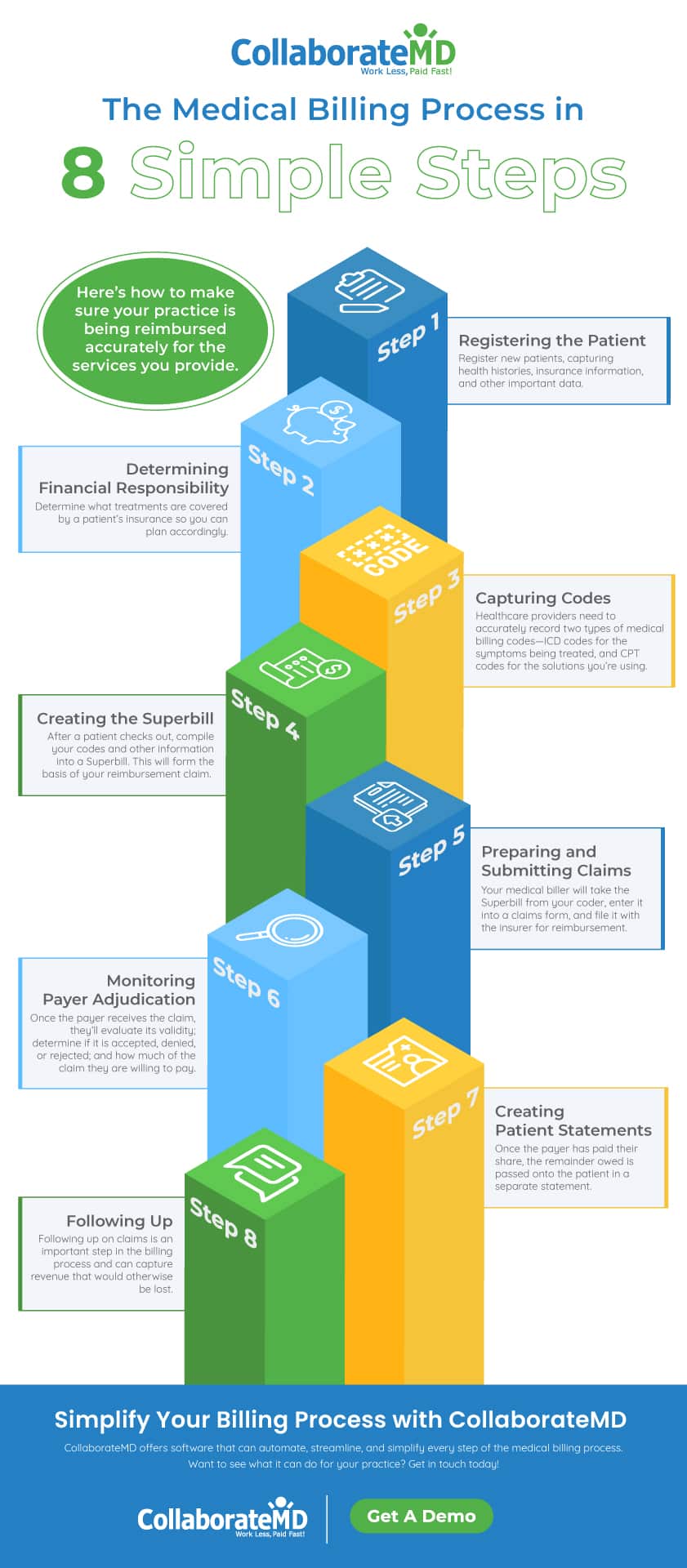
What Is the Medical Billing Process? Steps Cycle Overview
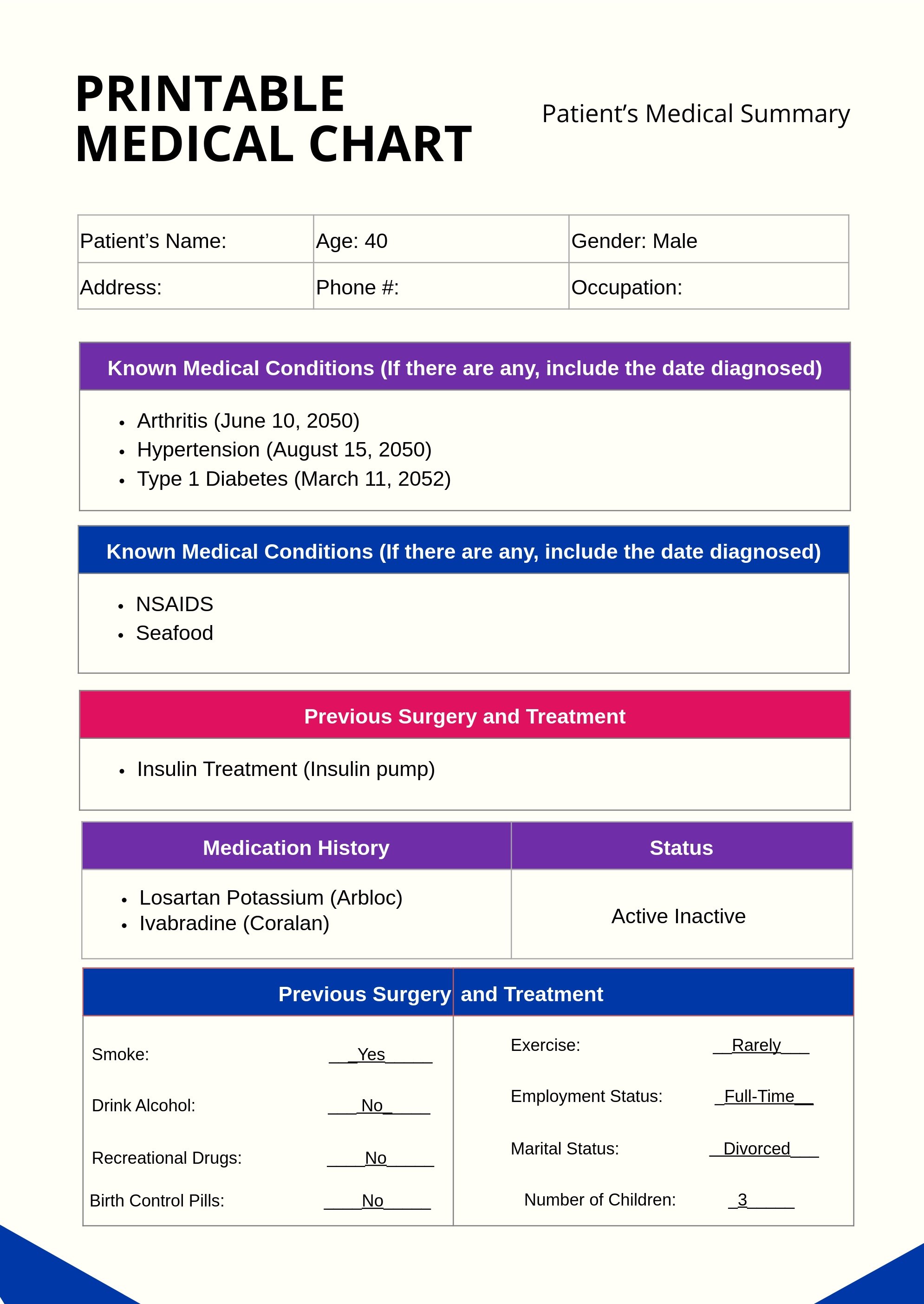
Free Medical Chart Templates Editable and Printable