Nitrosamine Risk Assessment Template
Here are some of the images for Nitrosamine Risk Assessment Template that we found in our website database.

Downloadable Nitrosamine Risk Assessment Template:

APIC Template For Report On Nitrosamine Risk Assessment Final PDF

Nitrosamine Impurities Quality Risk Management 2022 Tech publish

Part II : Nitrosamine Risk Assessment Questionnaire

N nitrosamine risk assessments for oral dosage forms: Nitrite content

Nitrosamine Risk Assessment for Human Drug Products

APIC Template For Report On Nitrosamine Risk Assessment Final PDF

Risk Assessment for Nitrosamine Impurities Veeprho

Risk Assessment Report On Nitrosamines Impurity Cefpodoxime Proxetil
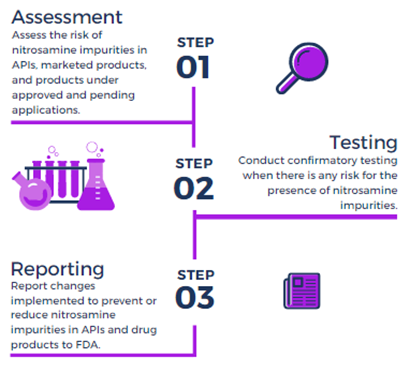
A Checklist For Risk Assessment Of Nitrosamine Impurities In Oral Solid

Nitrosamine Risk Assessment Guide for Your Drug Product

Risk Assessment and Mitigation of Nitrosamine Impurities

Table A 1 from Risk assessment of N‐nitrosamines in food Semantic Scholar

(PDF) Current Threat of Nitrosamines in Pharmaceuticals and Scientific
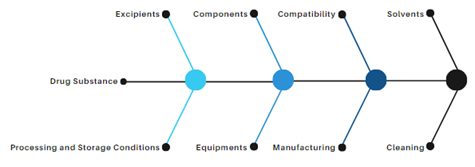
A Checklist For Risk Assessment Of Nitrosamine Impurities In Oral Solid

Navigating Nitrosamine Impurities: In Silico Risk Assessment

Nitrosamine Risk Assessment in Pharmaceuticals Industrial Pharmacist

New Nitrosamines Risk Assessment Deadlines Updates

Nitrosamine Risk Assessments in Oligonucleotides Pharma Excipients

FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of
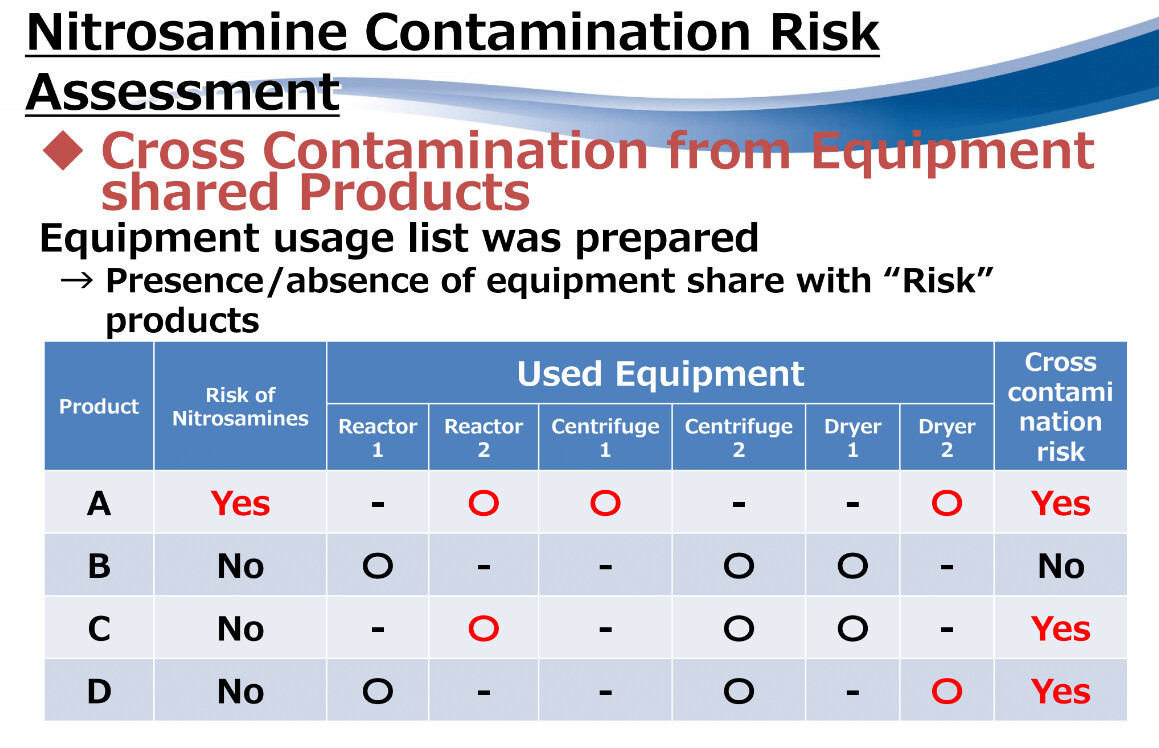
Nitrosamine Risk on shared equipment? Risk Assessment Strategy
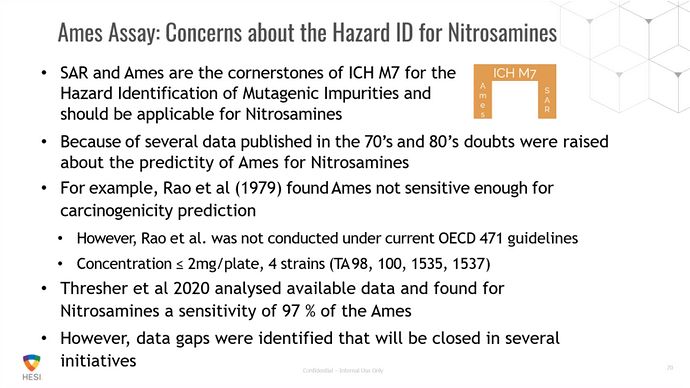
FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of

Nitrosamines in Pharmaceuticals: Toxicity Risk Analysis Chemistry
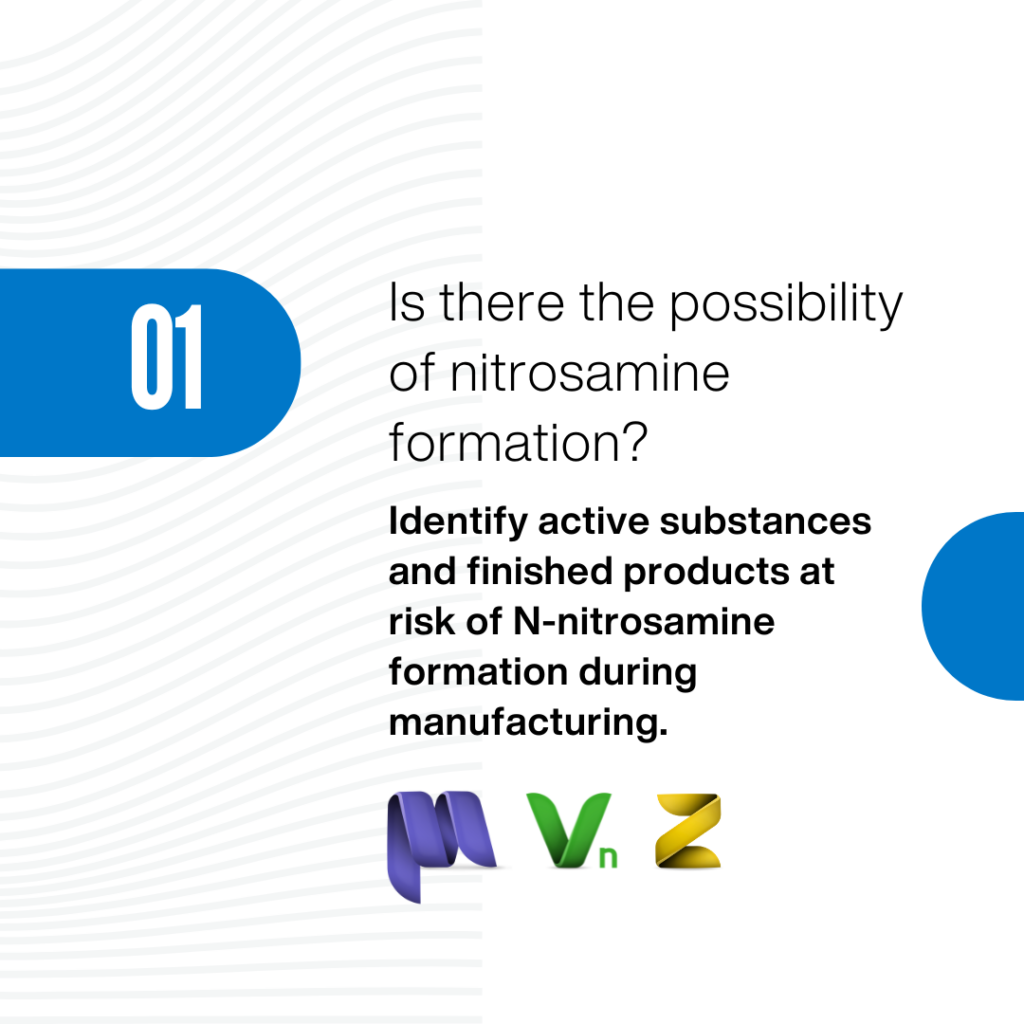
Navigating Nitrosamine Impurities: In Silico Risk Assessment
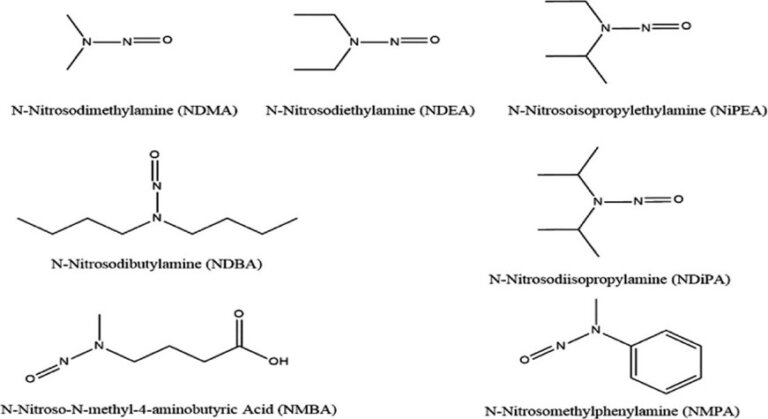
Risk assessment strategy for nitrosamine drug substance related
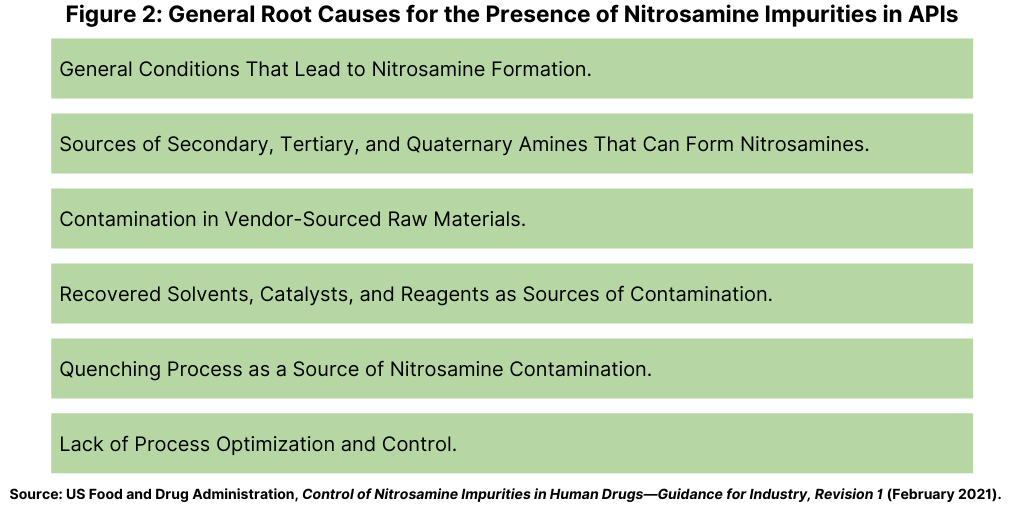
FDA Updates Risk Mitigation for Nitrosamine Impurities DCAT Value
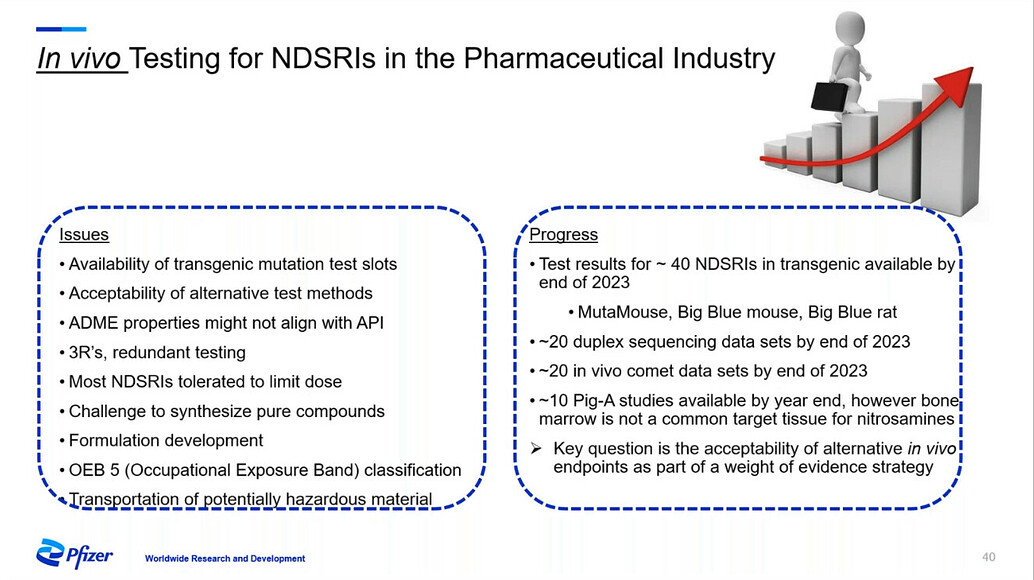
FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of
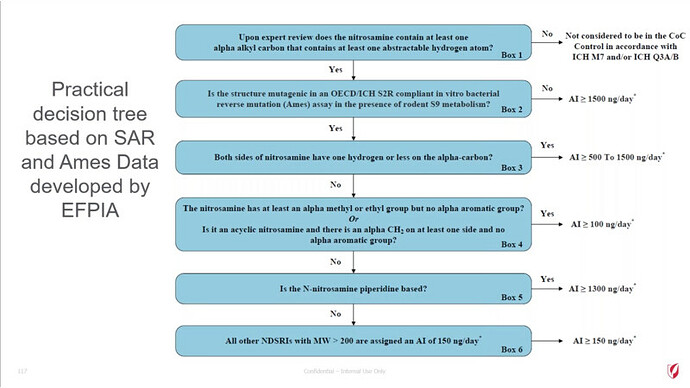
FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of

Guidance Nitrosamine Impurities Medications PDF Risk Assessment Risk
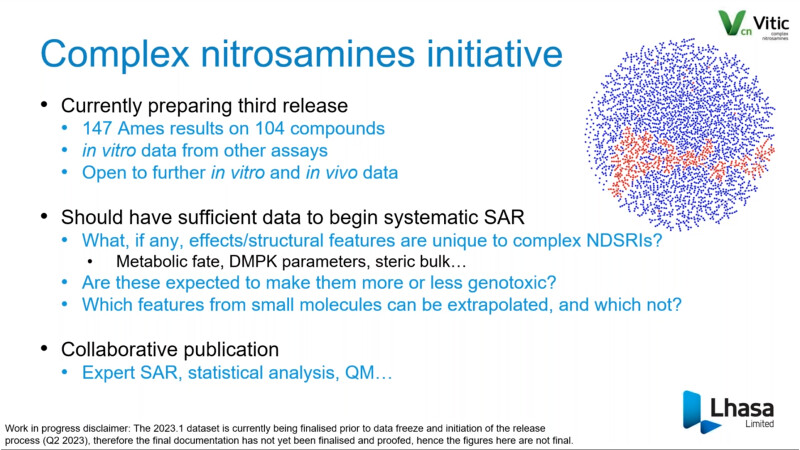
FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of

Nitrosamine Risk Assessment for Human Drug Products

Risk Assessment and Corrective Actions for Nitrosamine Impurities YouTube
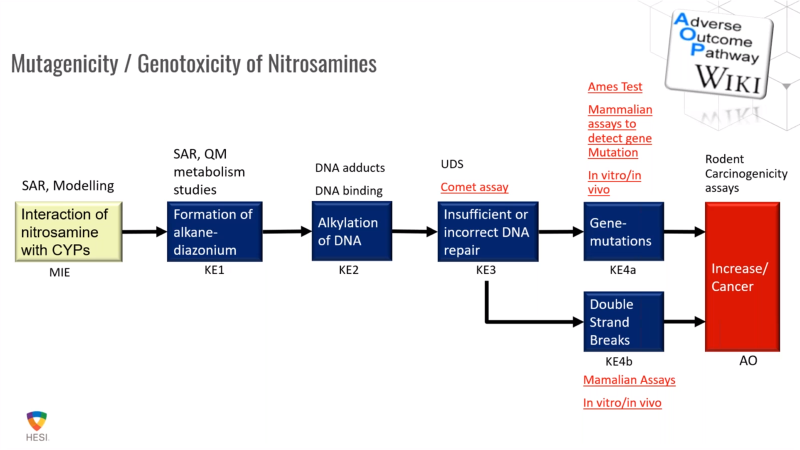
FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of

20 Tips For Nitrosamine Impurity Assessment Lhasa Limited

Nitrosamines Risk Assessments for BASF s pharmaceutical excipients
Nitrosamine Impurities Testing and Analysis ResolveMass Laboratories Inc

Nitrosamines in Pharmaceuticals: Toxicity Risk Analysis Chemistry

(PDF) Nitrosamine Impurities in Drug Substances and Drug Products

A deep dive into Nitrosamine impurities in APIs and drug products Veeprho
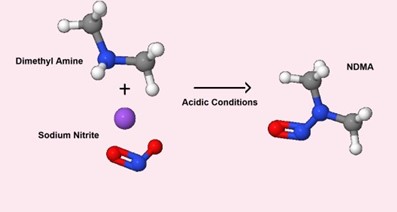
A Checklist For Risk Assessment Of Nitrosamine Impurities In Oral Solid

APIC Template For Report On Nitrosamine Risk Evaluation Update QA Rev12

Nitrosamine Contamination in Pharmaceuticals: A Comprehensive Review on

Risk assessment Batch testing requirements Nitrosamine impurities
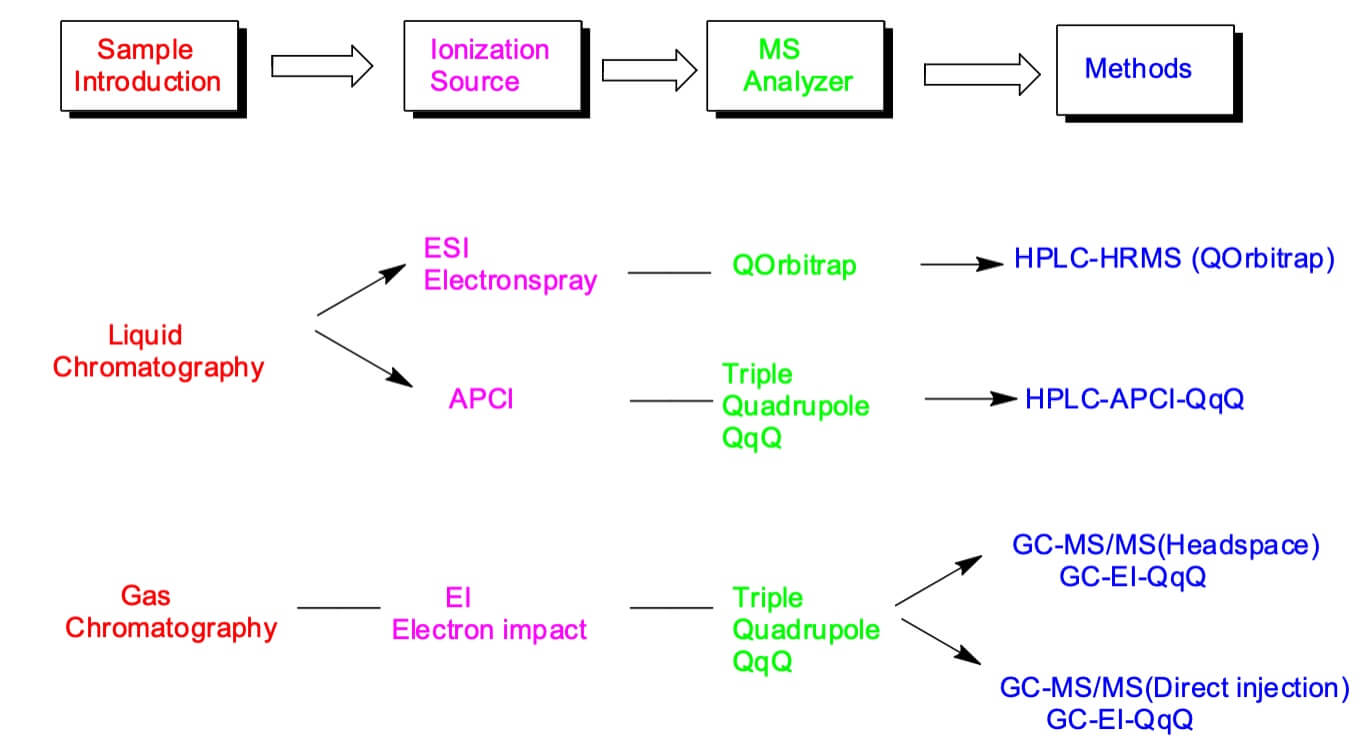
Nitrosamine Impurity Analysis Emery Pharma

Formation of N Nitrosamine Drug Substance Related Impurities in
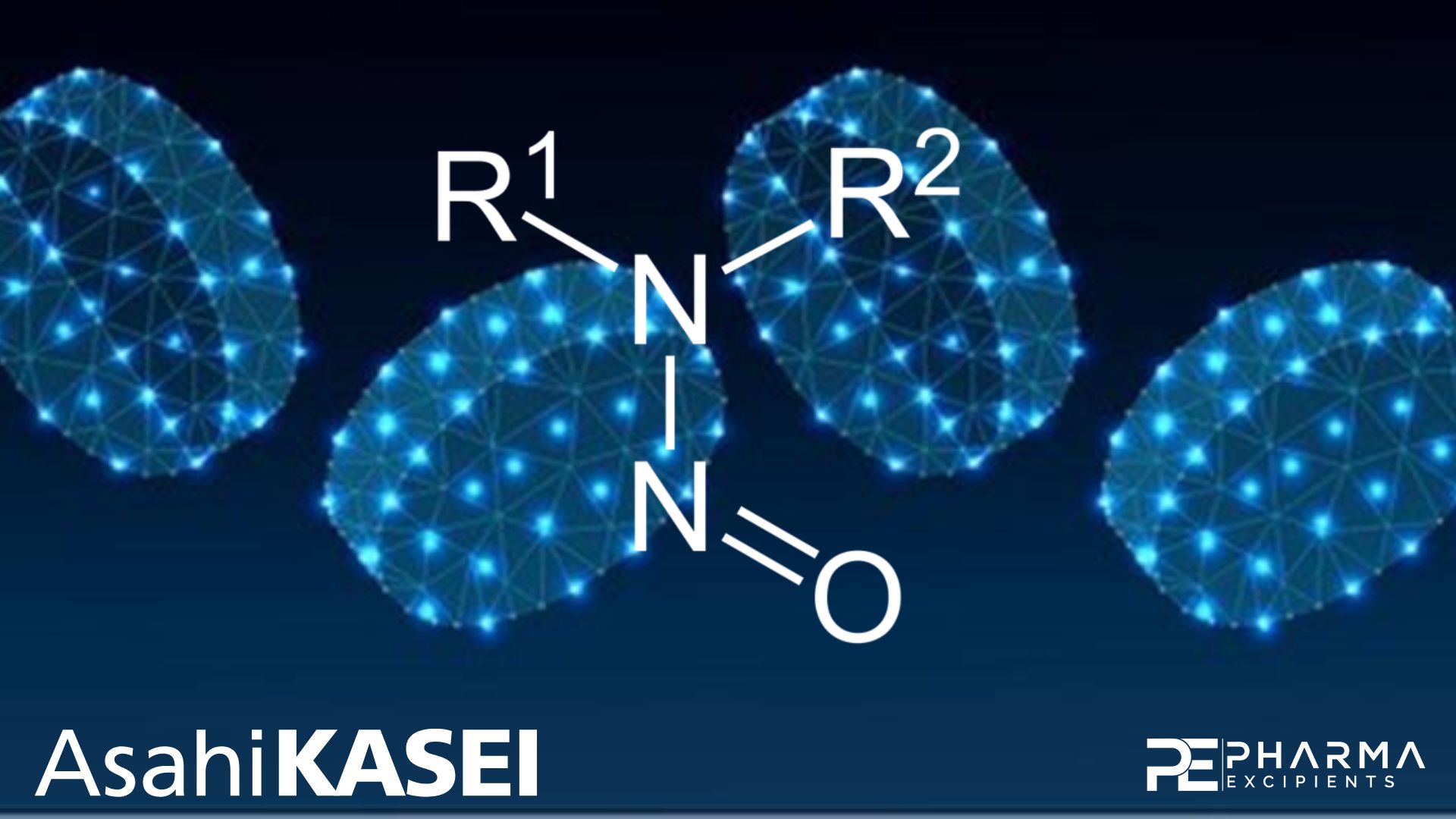
N nitrosamine risk assessments for oral dosage forms: Nitrite content

Nitrosamines In Human Medicinal Products PDF
FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of

Nitrosamine Impurities: API Pharmaceutical Products

High Sensitivity Quantitation of Nitrosamine Genotoxic Impurities: LC

Nitrosamine Impurity Risk Assessment Lhasa Limited

Nitrosamines risk assessment: step 2 YouTube

APIC Template For Report On Nitrosamine Risk Assessment Final PDF
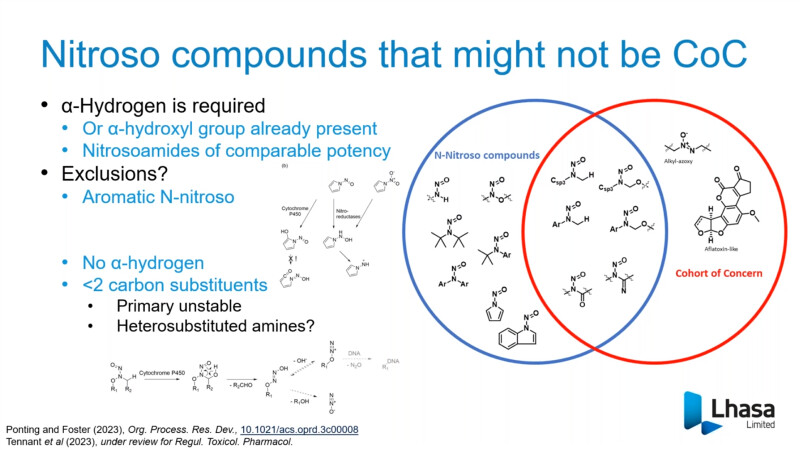
FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of
FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of
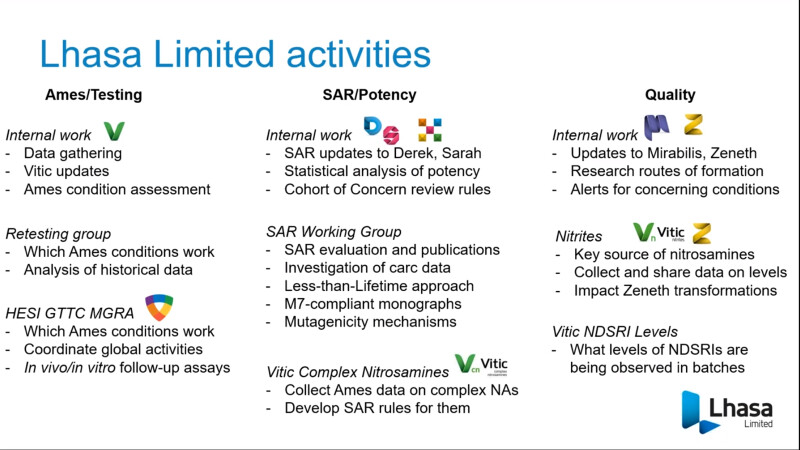
FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of

Risk Assessment for Nitrosamine Impurities YouTube
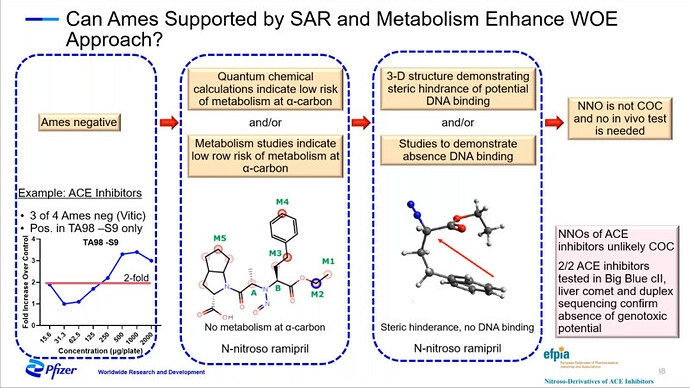
FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of

FDA/HESI Research Roadmap Planning on Hazard and Risk Assessment of
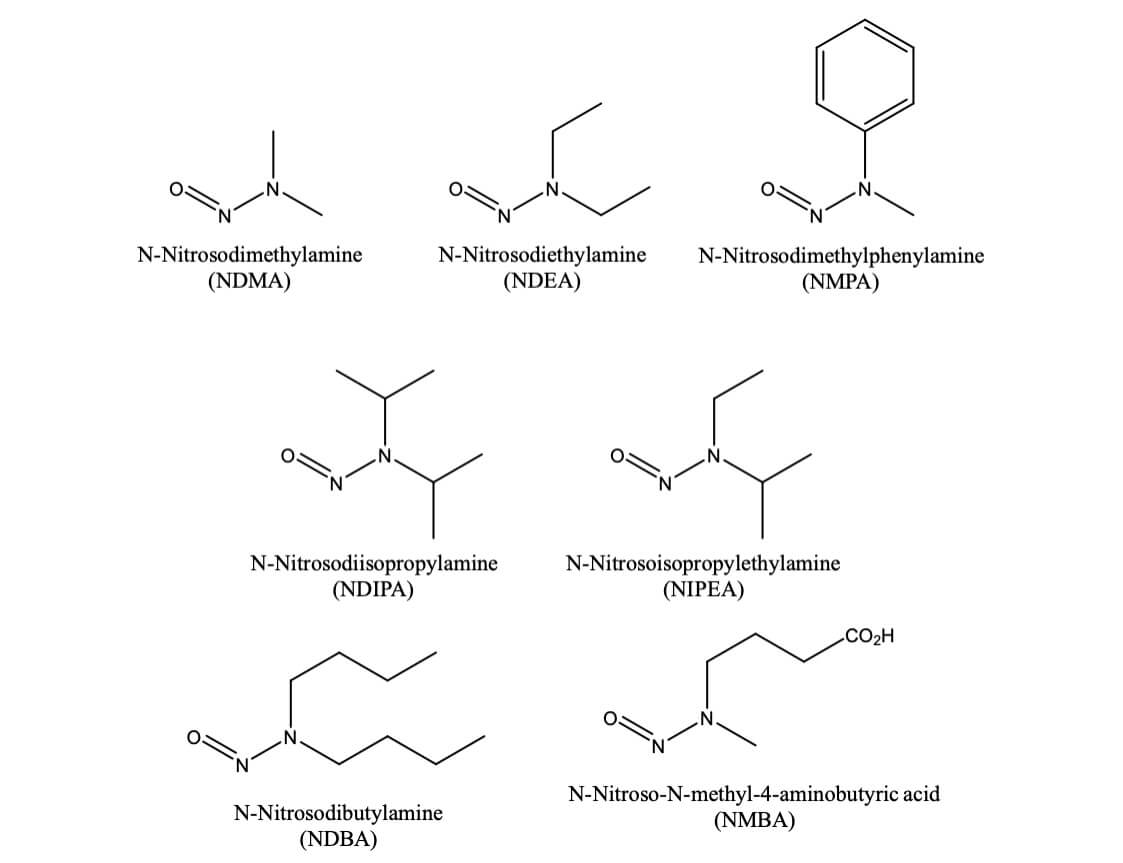
Nitrosamine Impurity Analysis Emery Pharma