Paragraph Iv Certification
Here are some of the images for Paragraph Iv Certification that we found in our website database.

Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX
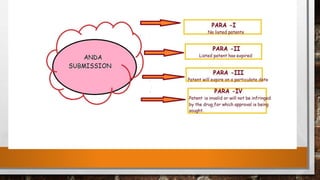
Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX
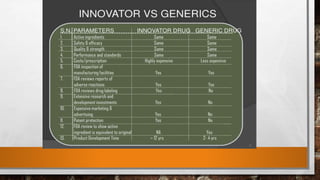
Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX
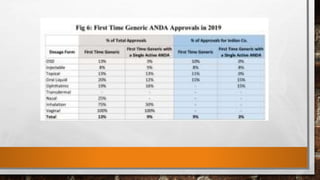
Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX
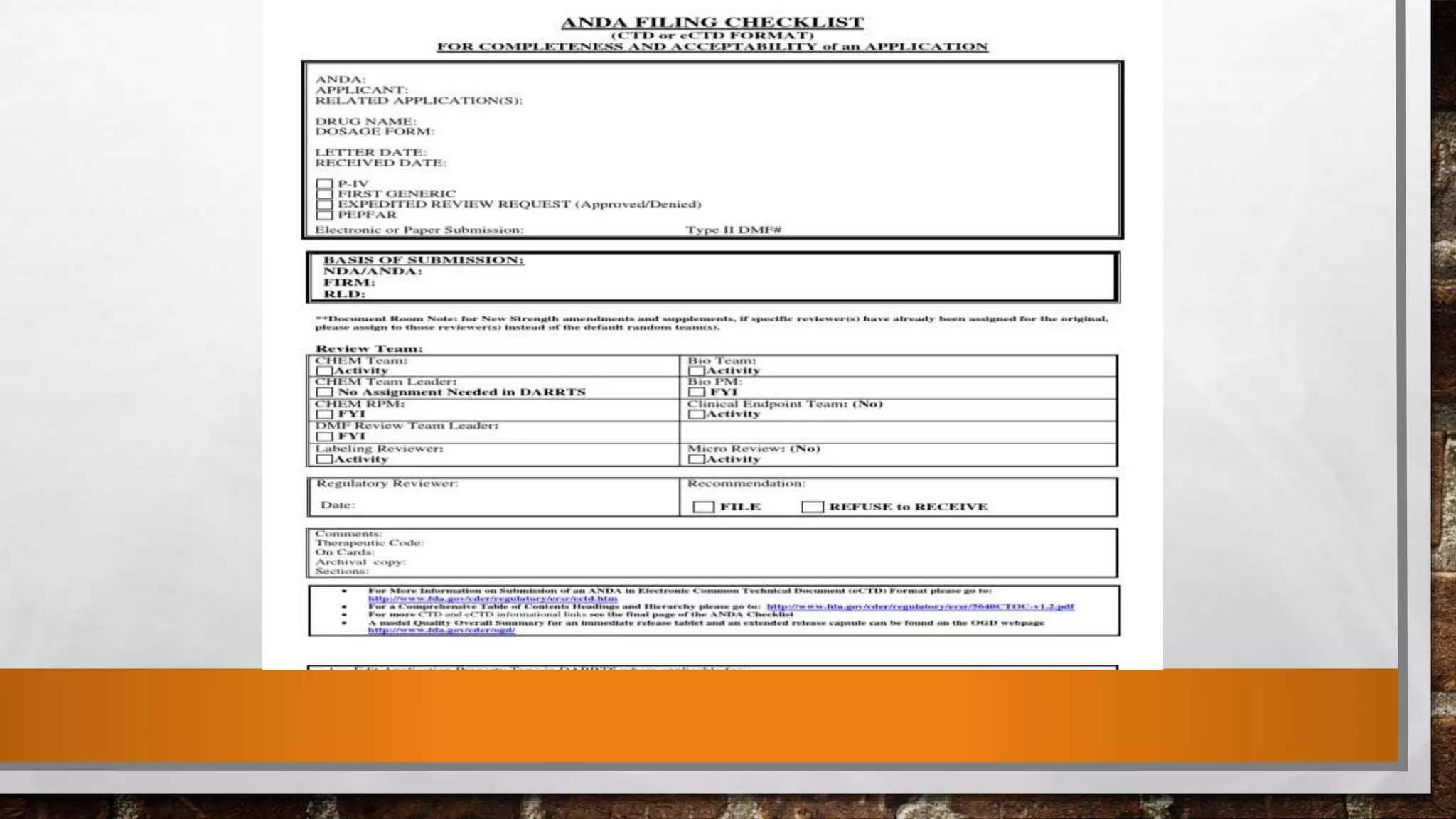
Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX
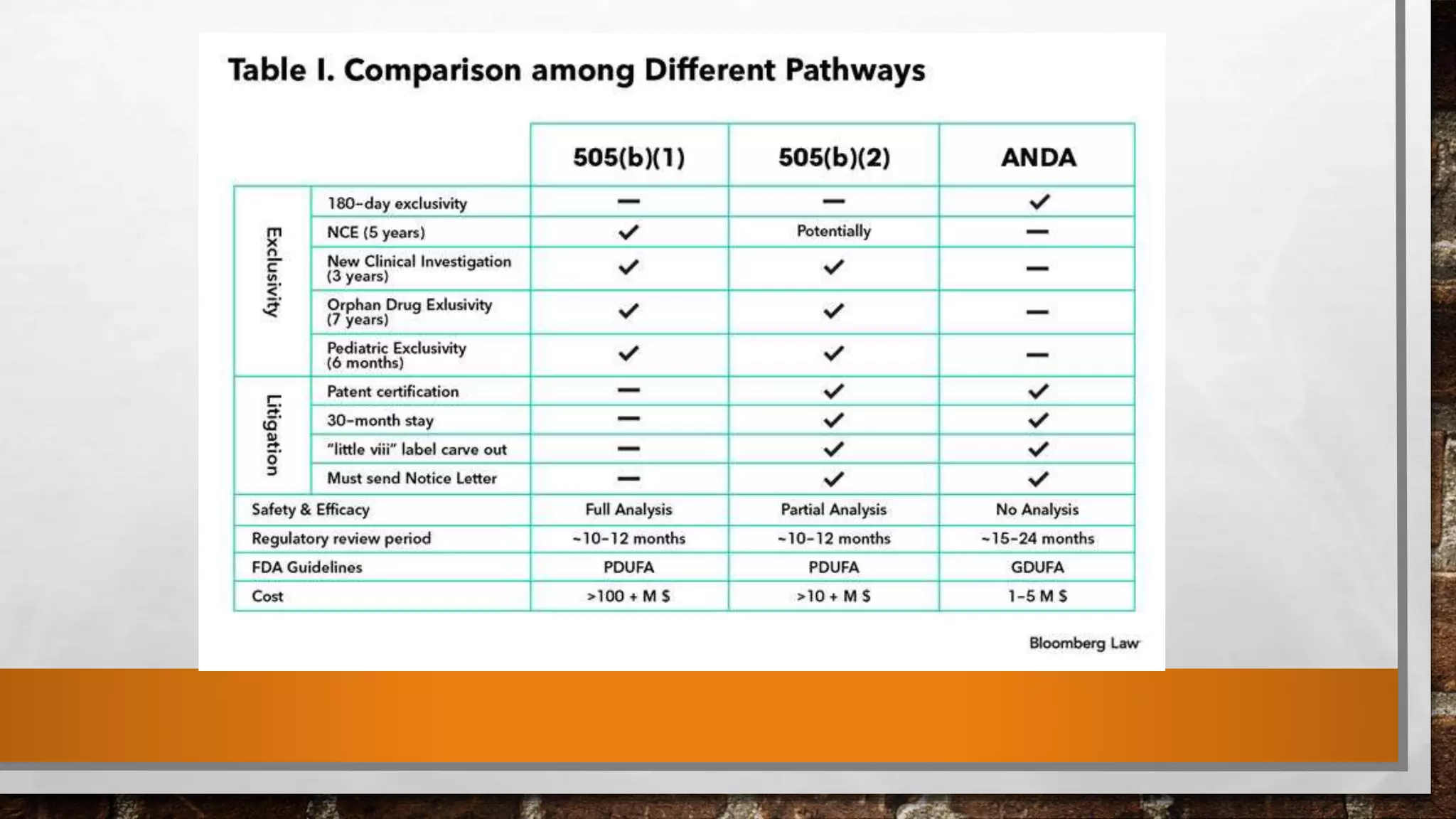
Anda submission and paragraph IV certification PPTX
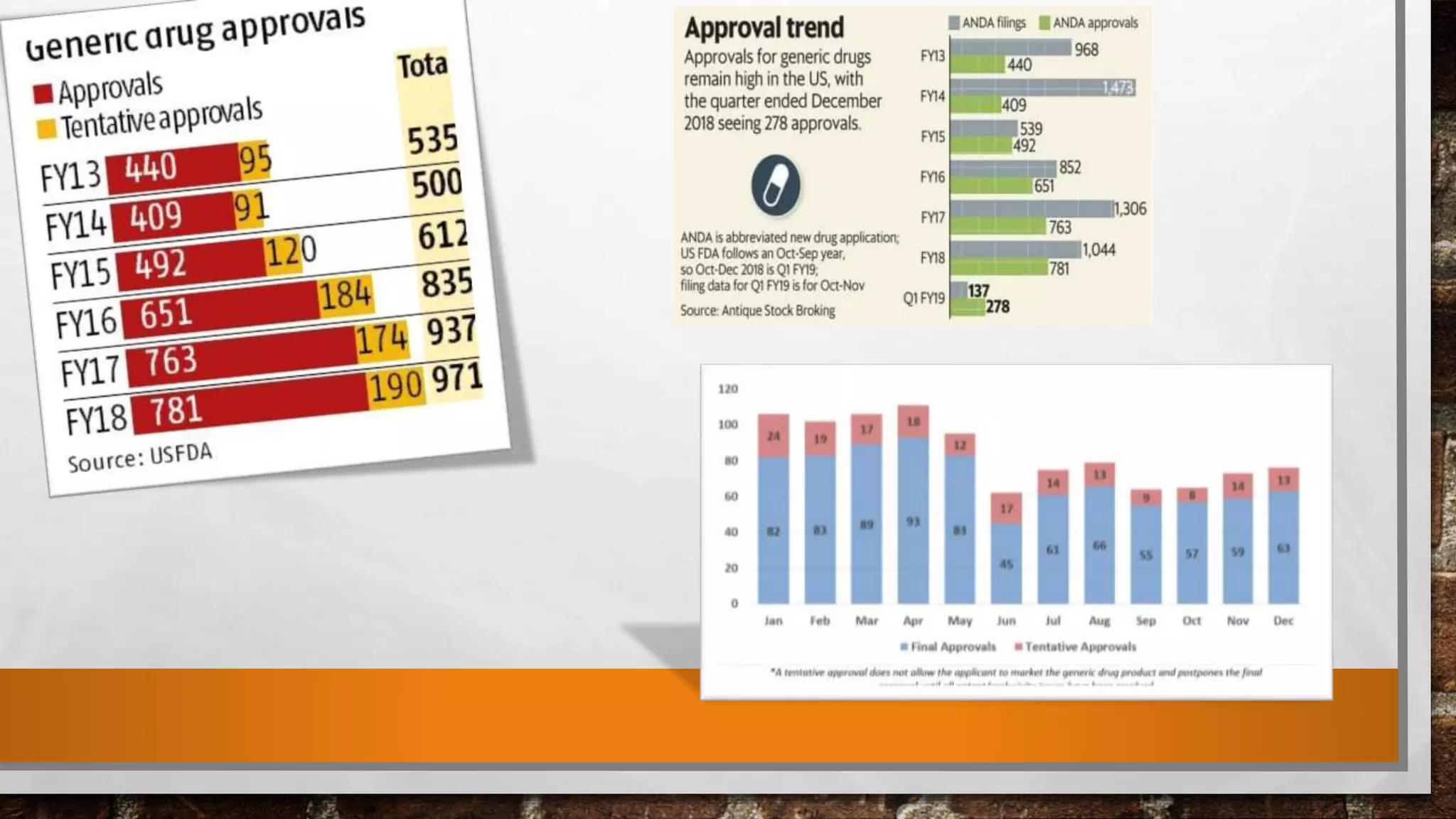
Anda submission and paragraph IV certification PPTX
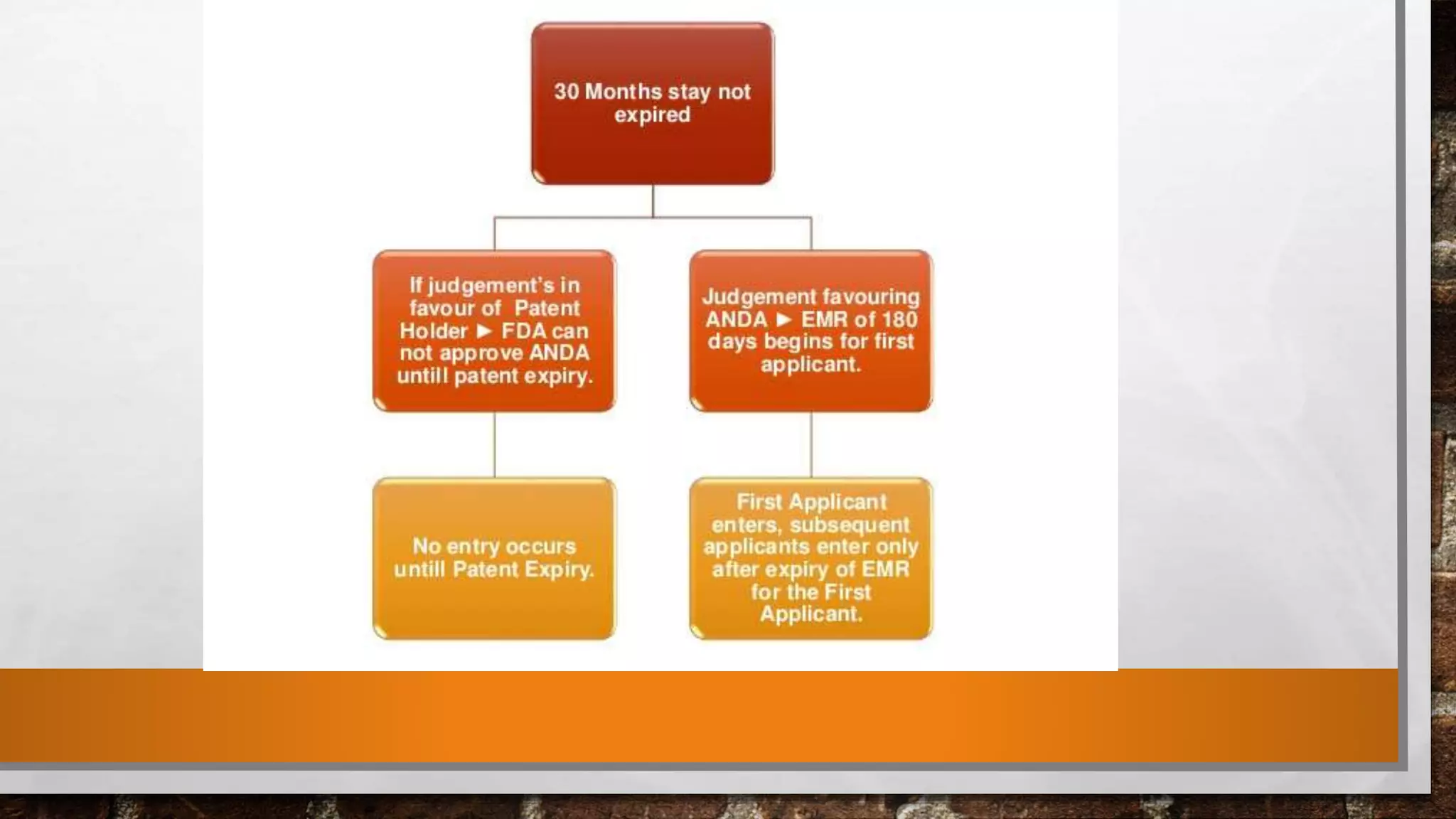
Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX
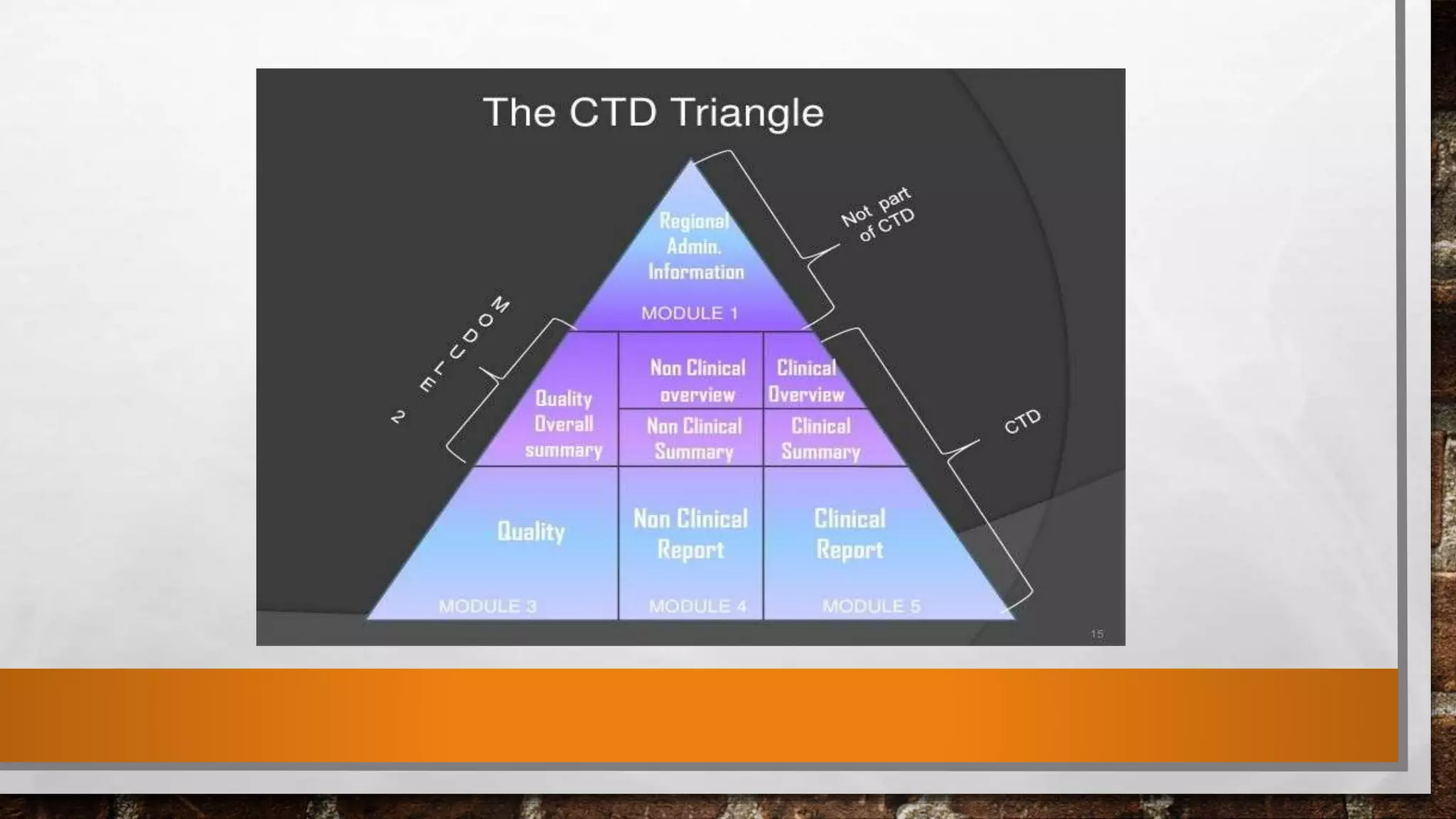
Anda submission and paragraph IV certification PPTX

Anda submission and paragraph IV certification PPTX Pharmaceutical

Anda submission and paragraph IV certification PPTX Pharmaceutical
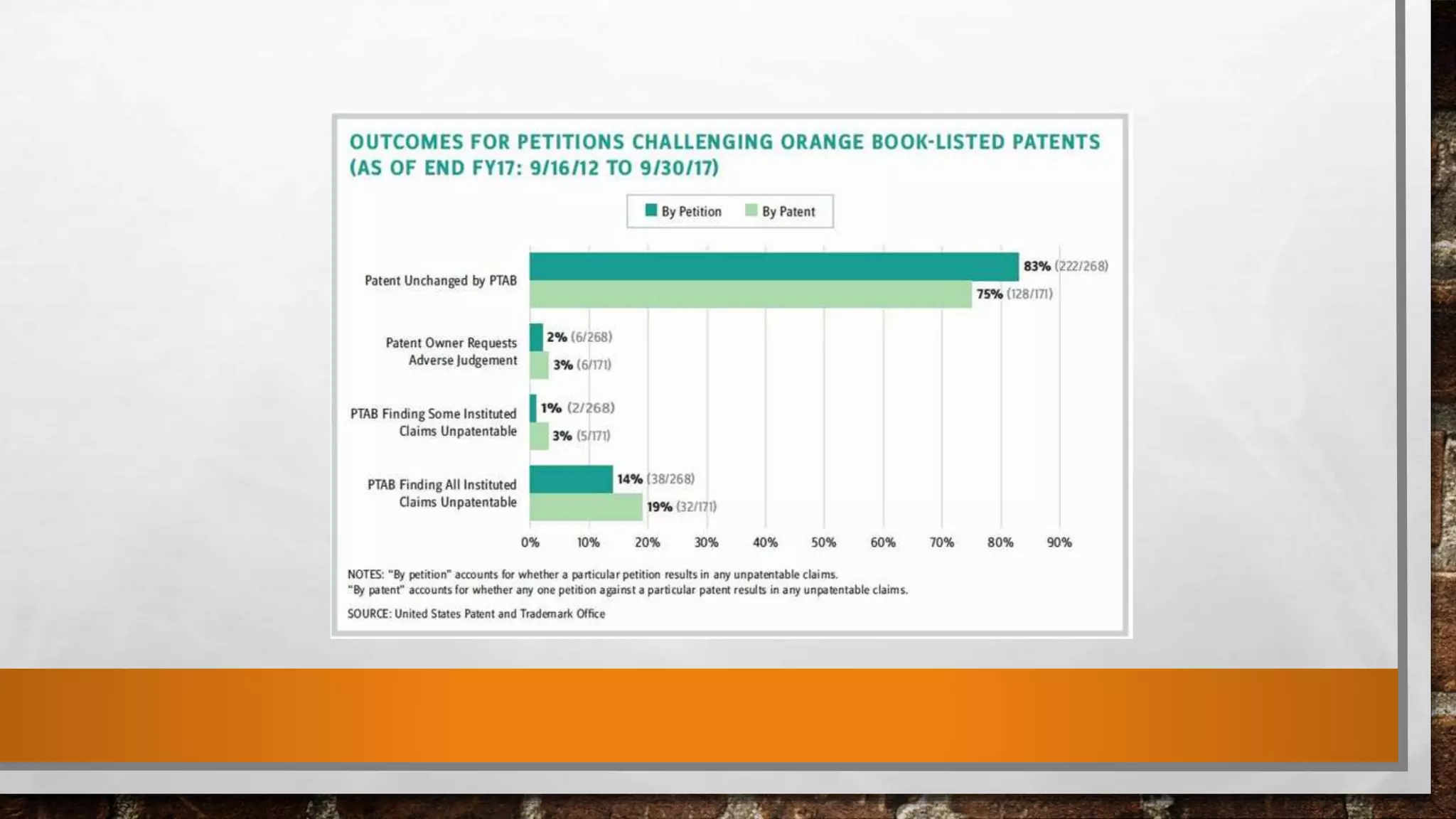
Anda submission and paragraph IV certification PPTX

First Biosimilar Making Paragraph IV Certification under China s New
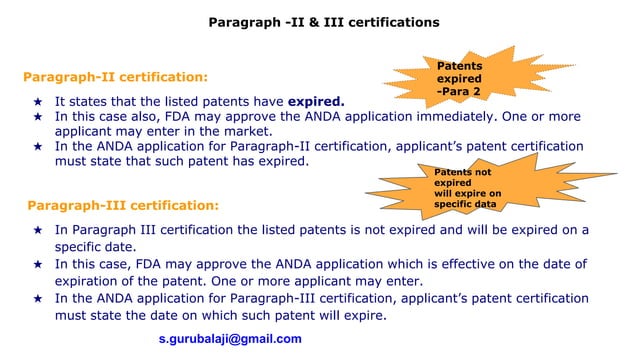
Paragraph 1 2 3 4 certification usfda PPTX

Paragraph 1 2 3 4 certification usfda PPTX
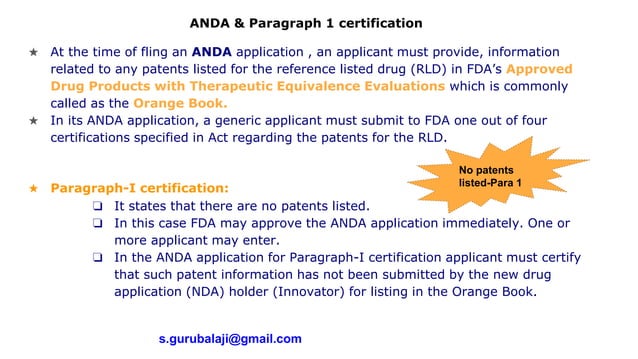
Paragraph 1 2 3 4 certification usfda PPTX

Paragraph 1 2 3 4 certification usfda PPTX

Paragraph 1 2 3 4 certification usfda PPTX

Paragraph 1 2 3 4 certification usfda PPTX

Paragraph IV

TRACK REGULATORY: USFDA : Paragraph IV Patent Certifications

(the quot Company quot ) issued a press release announcing its receipt of a
Paragraph IV Certifications Last 4 Plus Years Lachman Consultant

Hatch waxman act and paragraph iv litigations PPTX
FDA enhances Paragraph IV Patent Certifications List Drug Store News

Hatch waxman act and paragraph iv litigations PPTX

Hatch waxman act and paragraph iv litigations PPTX

Hatch waxman act and paragraph iv litigations PPTX

Hatch waxman act and paragraph iv litigations PPTX

4 New Paragraph IV Certifications Listed by FDA Lachman Consultant

4 New Paragraph IV Certifications Listed by FDA Lachman Consultant

What are Paragraph IV Patent Certifications?

NGE Attends American Conference Institute s 10th Annual Paragraph IV

(PDF) International Journal of Drug Regulatory Affairs Open Access to

(PDF) International Journal of Drug Regulatory Affairs Open Access to
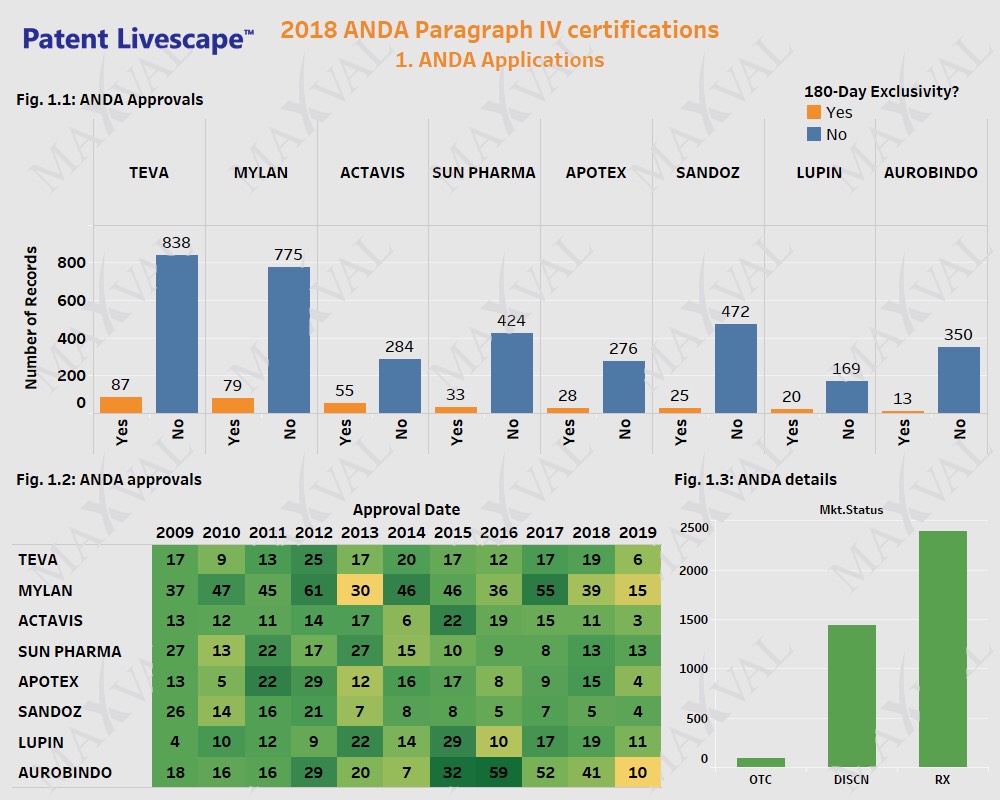
Generic Players to Benefit from Paragraph IV Approvals amidst FDA s

About Us Sigmapharm Laboratories LLC

About Us Sigmapharm Laboratories LLC

About Us Sigmapharm Laboratories LLC

About Us Sigmapharm Laboratories LLC
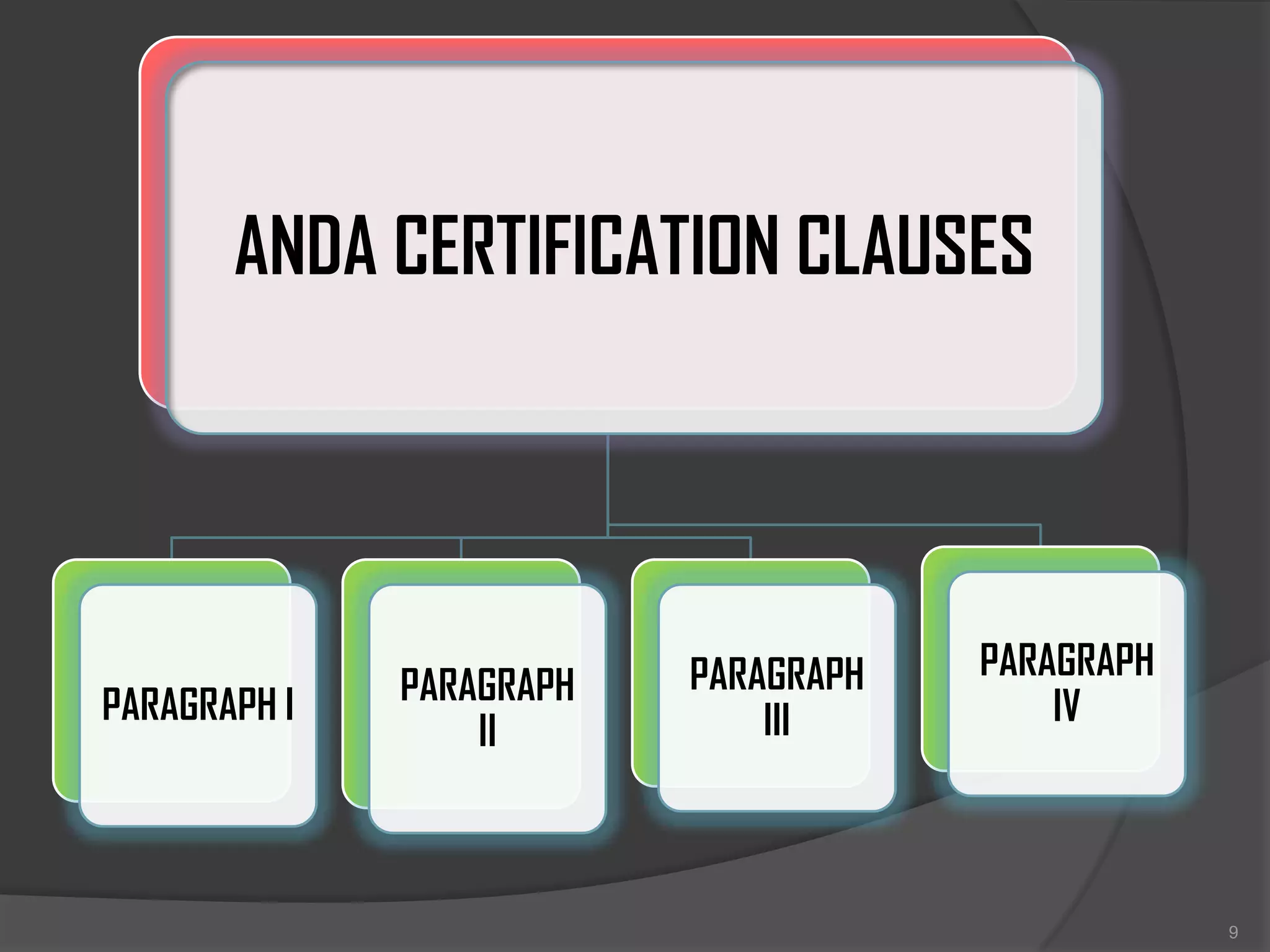
ANDA filing PPTX

Slide 21

Abbreviated New Drug Application (ANDA) pptx

Hatch waxman act amendments ppt PPTX