Regulatory Strategy Template For Medical Devices
Here are some of the images for Regulatory Strategy Template For Medical Devices that we found in our website database.

The Regulatory Process For Medical Devices PDF PDF Medical Device

Global Medical Device Regulatory Strategy Second Edition Toc PDF

Medical Device Regulatory Process Chart COMBINED PDF Medical Device

Regulatory Strategy Template For Medical Devices

Regulatory Strategy Template For Medical Devices

Regulatory Strategy Template For Medical Devices

Regulatory Strategy Template For Medical Devices
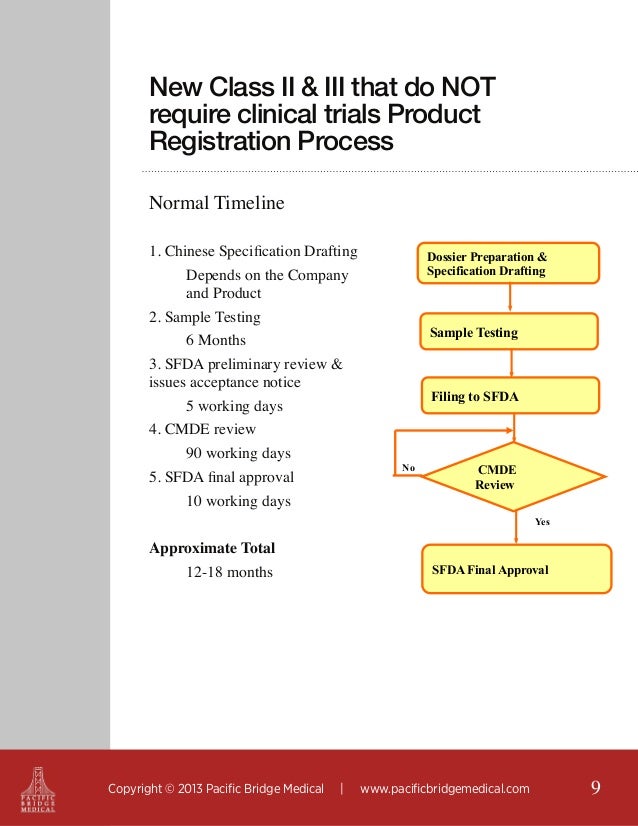
Regulatory Strategy Template For Medical Devices

Regulatory Brochure PDF Medical Device Quality Management System

Regulatory Strategy Medical Devices and Other Health Technologies AAPS

Regulatory Strategy Medical Devices and Other Health Technologies AAPS

Regulatory Compliance Strategy for Medical Devices Corpbiz

The Complete Regulatory Pathway for Medical Devices:
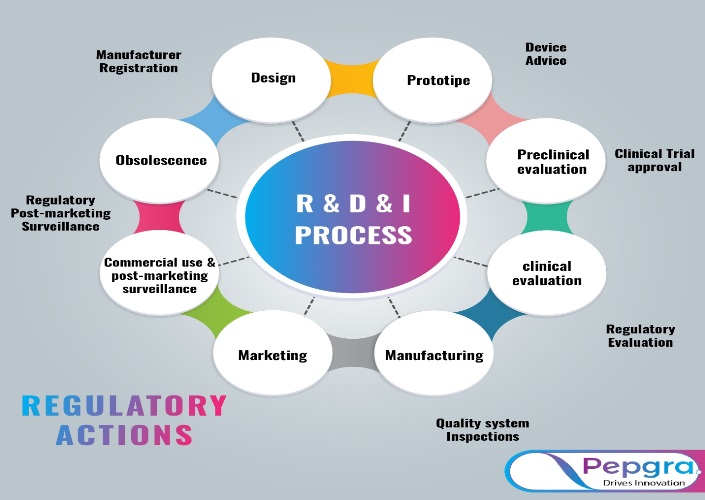
Regulatory Problems about Biomaterials and Medical Devices pepgra

How to Develop a Robust Regulatory Strategy for Medical Devices Step

How to Develop a Robust Regulatory Strategy for Medical Devices Step

Regulatory strategy for medical devices Medical Device Network

How to Communicate Your Regulatory Strategy to Medical Device Investors

3 things to consider on your medical device regulatory strategy post

Regulatory Intelligence for Medical Devices: Complete Guide

The 4 key principles of a smart regulatory strategy for medical devices

Effective Regulatory Compliance Strategy for Medical Devices
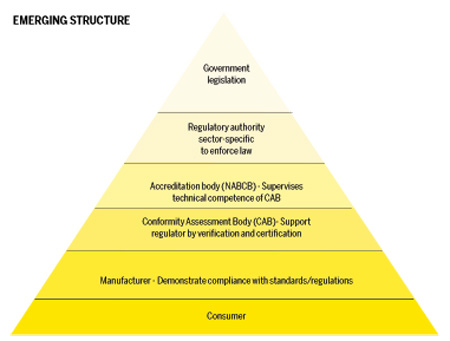
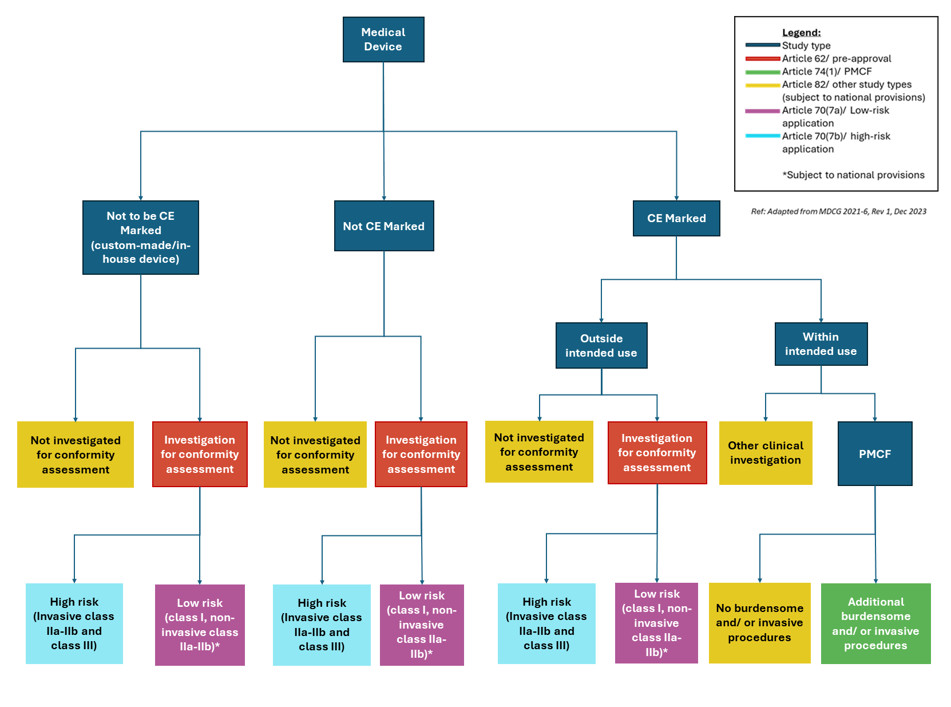
Regulatory framework for medical devices Express Healthcare

Medical Device Regulatory Strategy Rico Solutions

A Regulatory Strategy for your Medical Device DOCX Healthcare
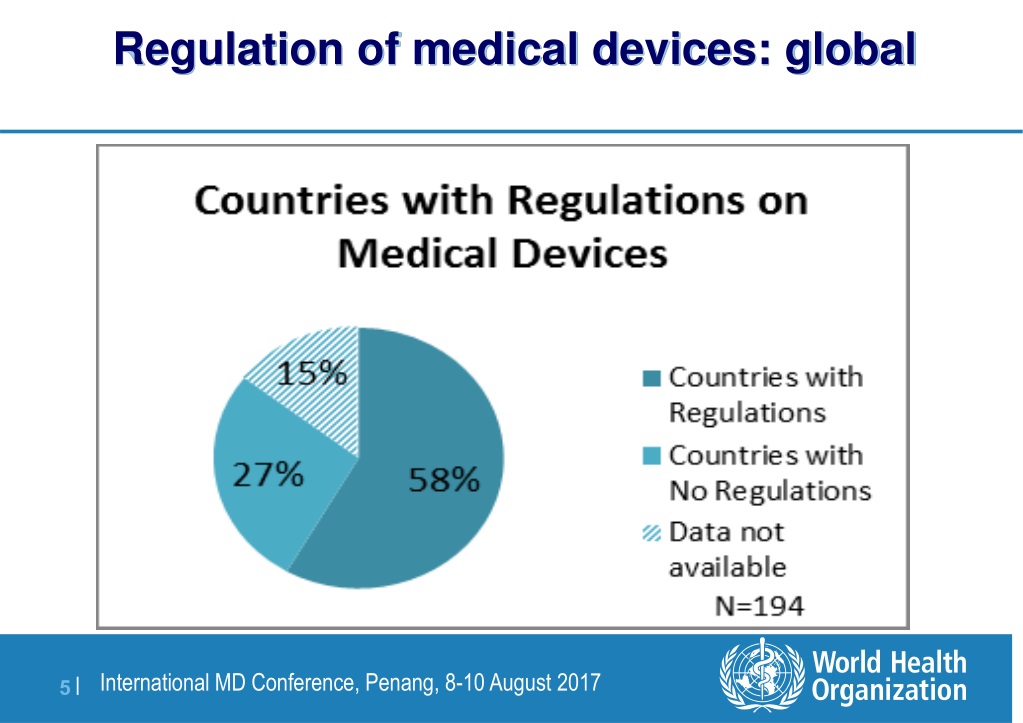
PPT WHO Global Regulatory Model Framework for medical devices

Navigating Regulatory Pathways for Implantable Medical Devices

Roadmap towards the future regulatory framework for medical devices

(PDF) Managing Medical Devices within a Regulatory Framework

Navigating Regulatory Pathways for Implantable Medical Devices

The Medical Device Regulatory Strategy Guide

Understanding regulatory pathways for medical devices TechTarget

(PDF) Regulatory Pathways for Medical Devices: Choosing the Right One
Understanding Regulatory Landscape: Singapore #39 s Medical Devices and

Developing a regulatory strategy: 6 good reasons

Can the regulatory strategy define medical device marketing strategy?

Medical Device Regulatory Affairs PPTX

Regulatory Plan Template prntbl concejomunicipaldechinu gov co
Whitepaper Overview of Medical Device Regulatory Affairs

Medical Device Regulatory Affairs PPTX

Medical Device Regulatory Affairs PPTX

Regulatory Roadmap announced for Medical Devices Specialist Quality

Medical Device Regulatory Framework PDF Medical Device Federal Food

IDEA Regulatory on LinkedIn: The answer to medical devices regulatory

Medical Device Regulatory Affairs pdf

Your Medtech Regulatory Strategy Is Your Market Strategy

Navigating regulatory challenges for medical device manufacturing

Tata Elxsi Medical Device Regulatory Compliance and Quality Assurance

Tata Elxsi Medical Device Regulatory Compliance and Quality Assurance

Analysis: Navigating an evolving medical device regulatory landscape

Medical Device Regulatory Services Medical Device Approvals Medical
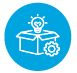
Regulatory Pathways for Medical Device Clinical Trials Lumis

Medical Device Regulatory Services UKCA CE and FDA Hardian Health
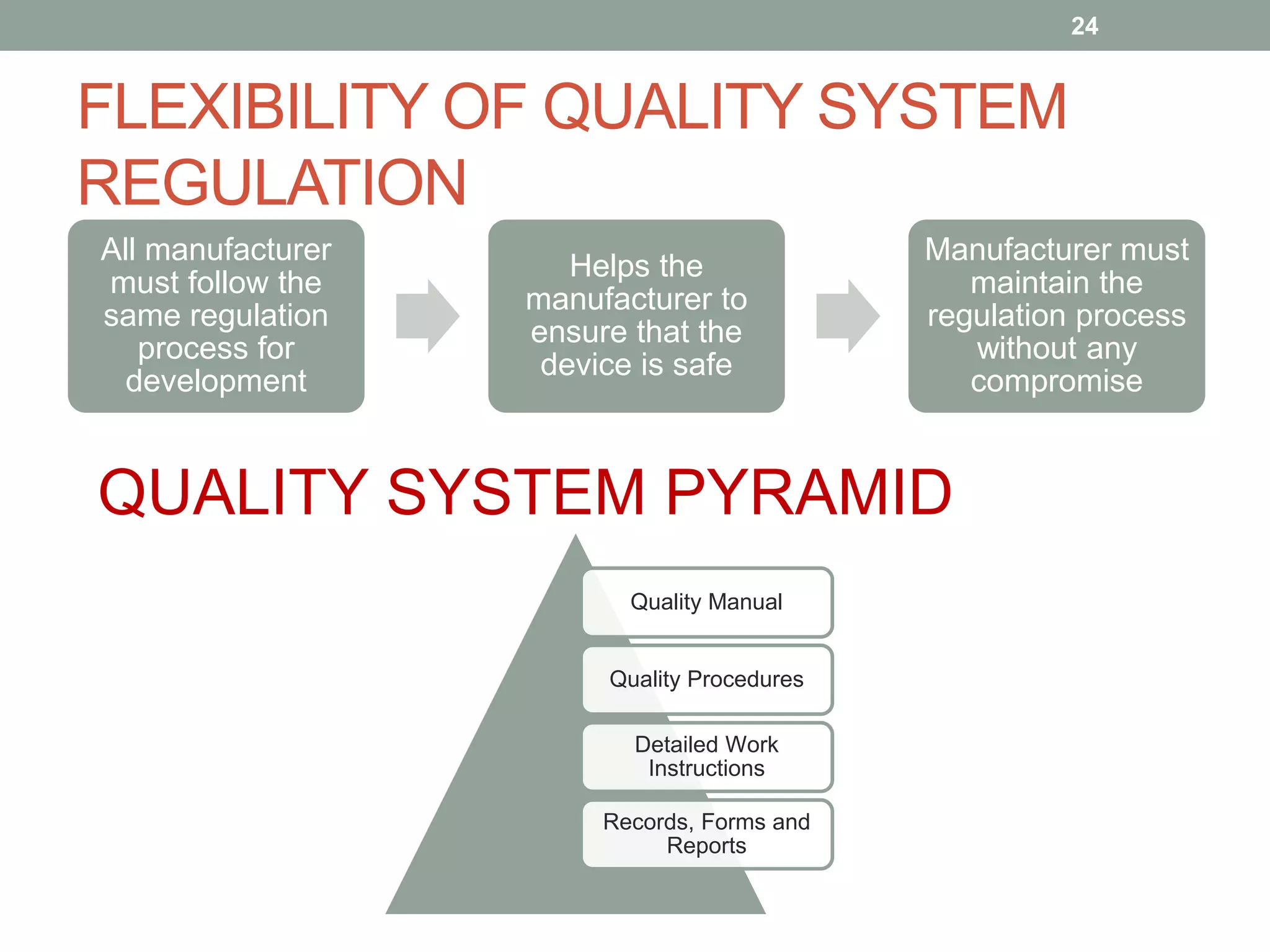
FDA regulation for medical devices PPTX

Medical Device Regulatory Affairs Coggle Diagram

Regulatory 101

Regulatory Strategy for Medical Devices: Global Market Planning

Regulatory Medical Device Innovation Center

Why is a regulatory strategy important for medical devices?